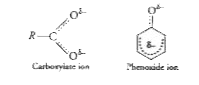
Text Solution
Verified by Experts
Topper's Solved these Questions
CARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise PART II QUESTIONS FOR ASSESSMENT (MCQ) |2 VideosCARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise PART II QUESTIONS FOR ASSESSMENT (VERY SHORT ANSWER TYPE QUESTIONS) |1 VideosCARBOXYLIC ACIDS
ARIHANT PUBLICATION|Exercise PART II QUESTIONS FOR PRACTICE (SHORT ANSWER TYPE II QUESTIONS)|1 VideosARYL DIAZONIUM SALTS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) 7 MARKS |2 VideosCHEMICAL KINETICS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Long Answer Type Questions)|8 Videos
Similar Questions
Explore conceptually related problems