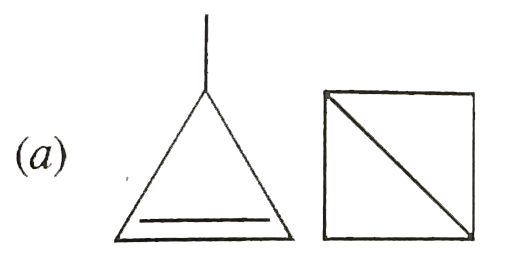
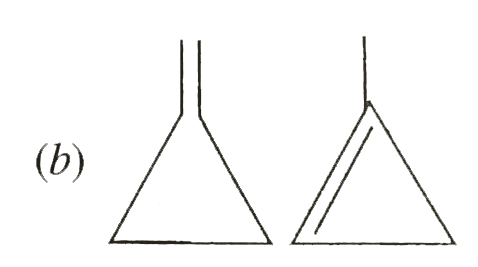
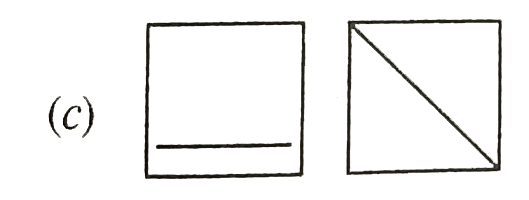
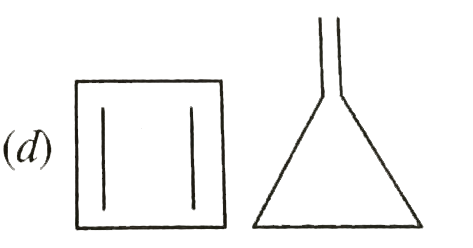
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which pair does not represent a cyclic compound of the formula C(4)H(6...
Text Solution
|
- Write all the acyclic and cyclic isomers of a compound having molecula...
Text Solution
|
- Which pair does not represent a cyclic compound of the formula C(4)H(6...
Text Solution
|
- The formula of hydrocarbon is C(4)H(6) ? To which family does it belon...
Text Solution
|
- You are given the following molecular formulae of some hydrocarbons: (...
Text Solution
|
- Give the possible cyclic isomers of formula C(6)H(12).
Text Solution
|
- Write all the acyclic and cyclic isomers (excluding stereoisomers) of ...
Text Solution
|
- The total number of cyclic isomers of formula C(6)H(12) is/are
Text Solution
|
- कौनसा युग्म C4 H6 अणु सूत्र वाले चक्रीय यौगिक को प्रदर्शित नहीं करता ह...
Text Solution
|