Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
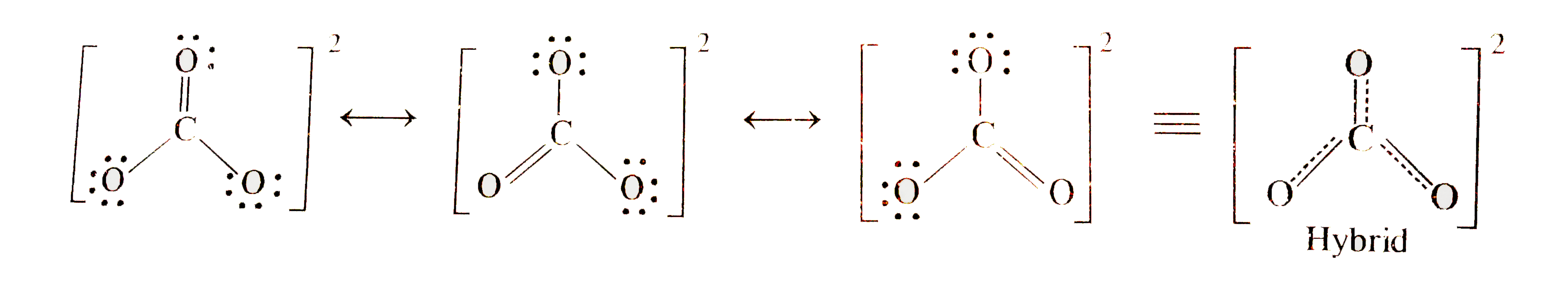
- Explain the structure of CO(3)^(2-) ion in terms of resonance (b) Ex...
Text Solution
|
- Explain the structure of CO(3)^(2-) ion in terms of resonance (b) Ex...
Text Solution
|
- Explain the structure of CO(2) molecule.
Text Solution
|
- Draw the resonating structures fo CO(2) molecule.
Text Solution
|
- CO(3)^(2-) आयन के संदर्भ में अनुनाद के भिन्न पहलुओं को स्पष्ट कीजिए ।
Text Solution
|
- अनुनाद तथा अनुनाद ऊर्जा को प्रभावित कीजिए । अनुनाद के लिए आवश्यक ...
Text Solution
|
- Write the resonating structures for the following molecules. CO(2)
Text Solution
|
- Write the resonating structures for the following molecules. CO(3)^(...
Text Solution
|
- Write the resonance structure of carbonate ion (CO(3)^(-2))
Text Solution
|