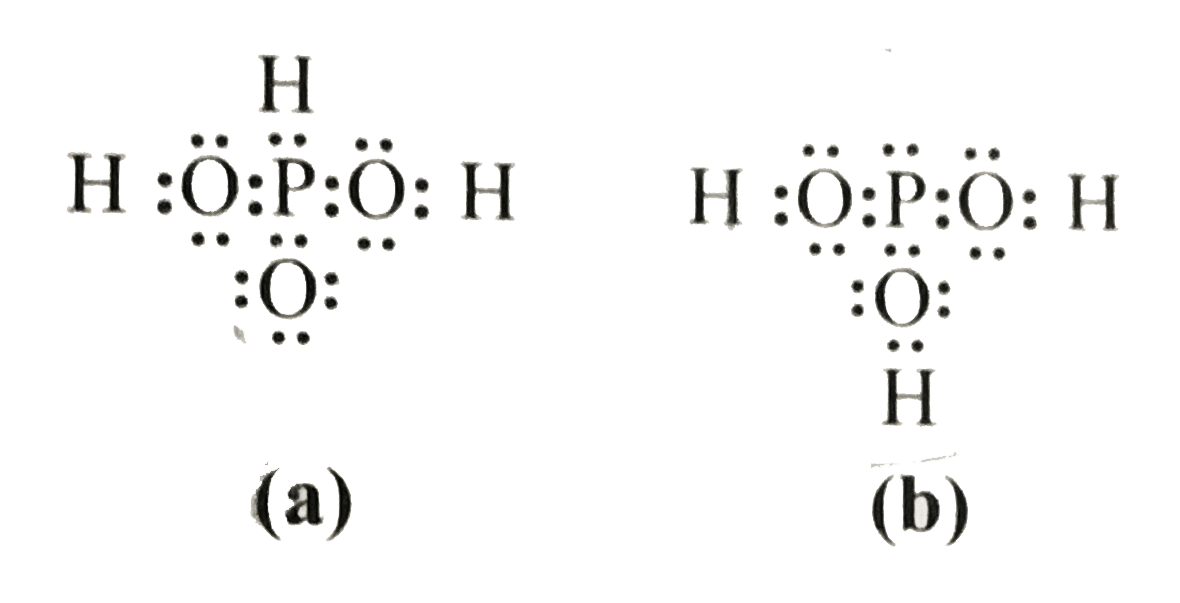Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- H(3)PO(3) can be represented by structure (a) and (b) shown below. Can...
Text Solution
|
- H(3)PO(3) can be represented by structure (a) and (b) shown below. Can...
Text Solution
|
- How is the reduction ability of H(3)PO(2) and H(3)PO(3) accounted on t...
Text Solution
|
- निचे दी गई संरचनाओं (1 तथा 2 ) द्वारा H(3)PO(3) को प्रदर्शित किया...
Text Solution
|
- नीचे दी गई संरचनाओं (1 तथा 2 ) द्वारा H(3)PO(3) को प्रदर्शित किया जा...
Text Solution
|
- नीचे दी गई संरचनाओं ( 1 तथा 2 ) द्वारा H(3)PO(3)को प्रदर्शित किया जा स...
Text Solution
|
- Give reasons for the following: H(3)PO(2) is a stronger reducing age...
Text Solution
|
- Draw the structures of the following: H(3)PO(2)
Text Solution
|
- Give reasons: H(3)PO(3) undergoes disproportionation reaction, but H(3...
Text Solution
|