Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
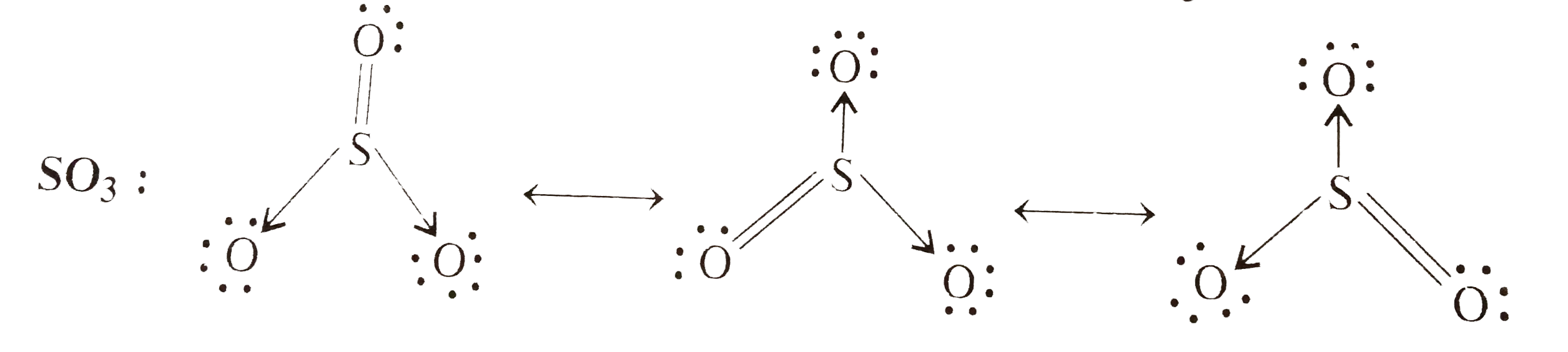
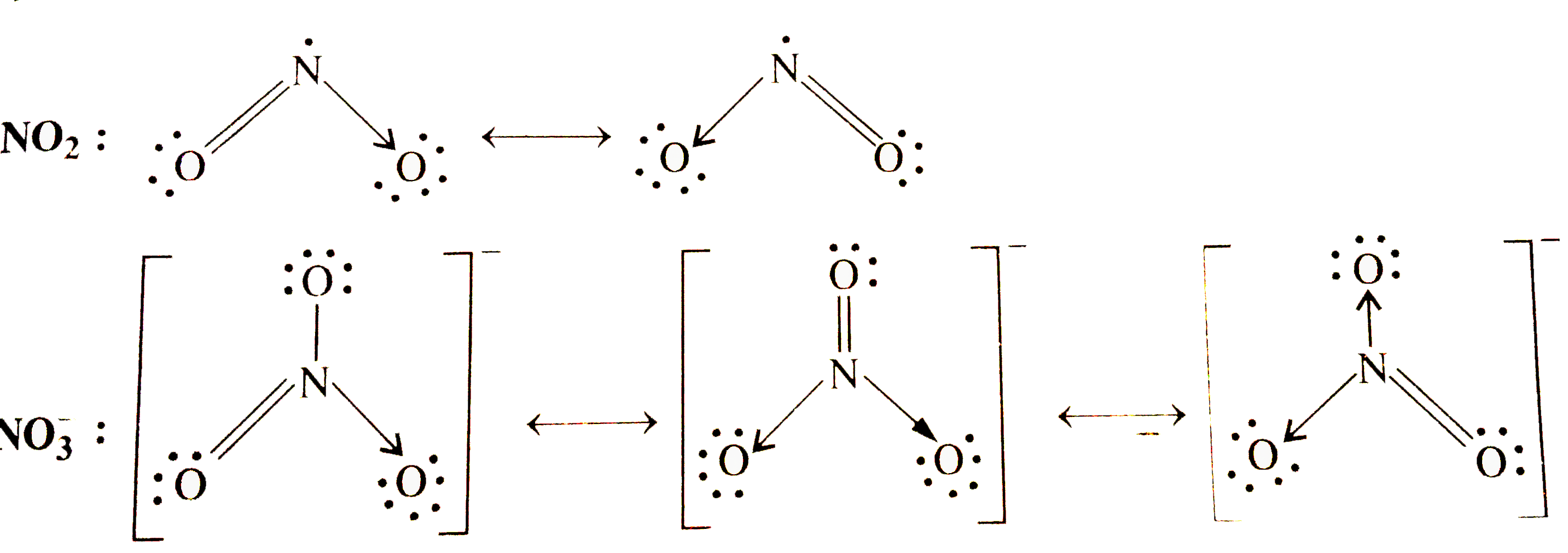
- Write the resonance structures for SO(3),NO(2), and NO(3)^(ө).
Text Solution
|
- Write the resonance structure of NO(2)^(Θ) (nitrite) and NO(3)^(Θ) (ni...
Text Solution
|
- Write the resonance structure of CO(3)^(2-) and HCO(3)^(ө).
Text Solution
|
- Wrtie the reasonance structure for SO(3) ,NO(2) and NO(3)^(-) .
Text Solution
|
- SO(3),NO(2) तथा NO(3)^(-) की अनुनादी संरचनाएँ लिखिए ।
Text Solution
|
- NO(2) तथा N(2)O(5) की अनुनाद संरचनाएँ लिखिये।
Text Solution
|
- NO(2) तथा N(2)O(5) कि अनुनादी संरचनाओं को लिखिए ।
Text Solution
|
- NO(2) तथा N(2)O(5) के अनुनादी संरचनाओं को लिखिए|
Text Solution
|
- SO(3),NO(2) तथा NO(3)^(-) की अनुनाद-संरचनाऍं लिखिए।
Text Solution
|