Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
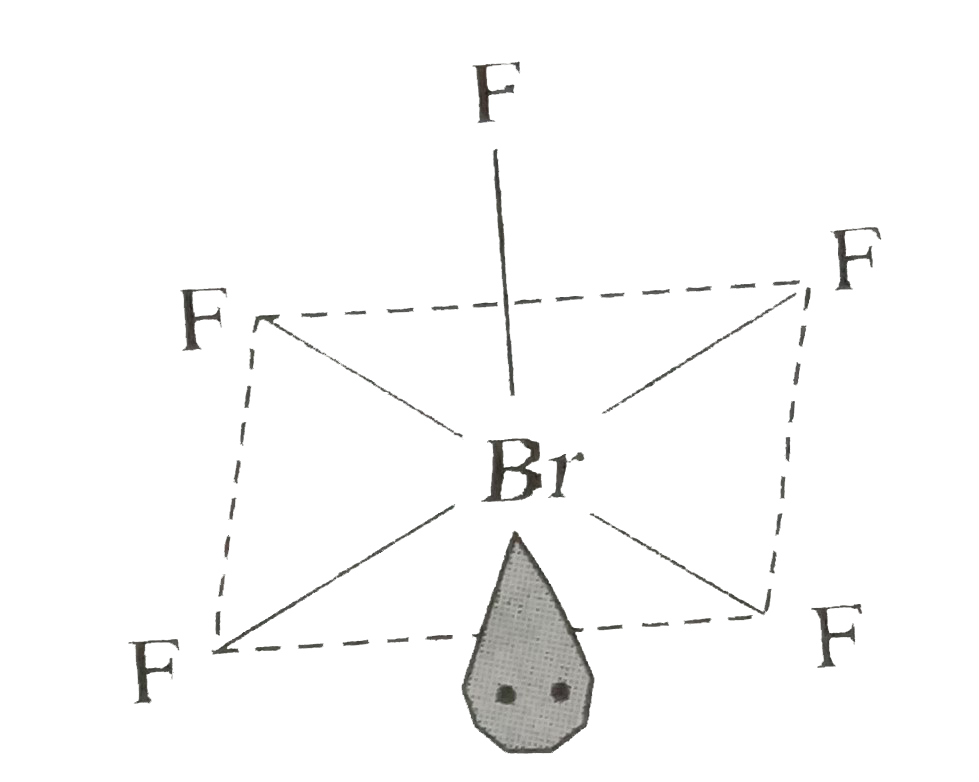
- Explain the shape of BrF(5)
Text Solution
|
- Using VSEPR theory draw the shape of PCI(5) and BrF(5) ?.
Text Solution
|
- Using VSEPR theory, draw the shape of PCL(5) and BrF(4).
Text Solution
|
- Explain the shape of BrF(5^.)
Text Solution
|
- Hybridisation and shape of BrF(5) is :
Text Solution
|
- Explain the shape of BrF(5^.)
Text Solution
|
- VSEPER सिद्धान्त के आधार पर BrF(3) की आकृति की व्यख्या कीजिए।
Text Solution
|
- Explain the structures of BrF(5)
Text Solution
|
- What is the shape of ClF(3),BrF(5),IF(7) molecules.
Text Solution
|