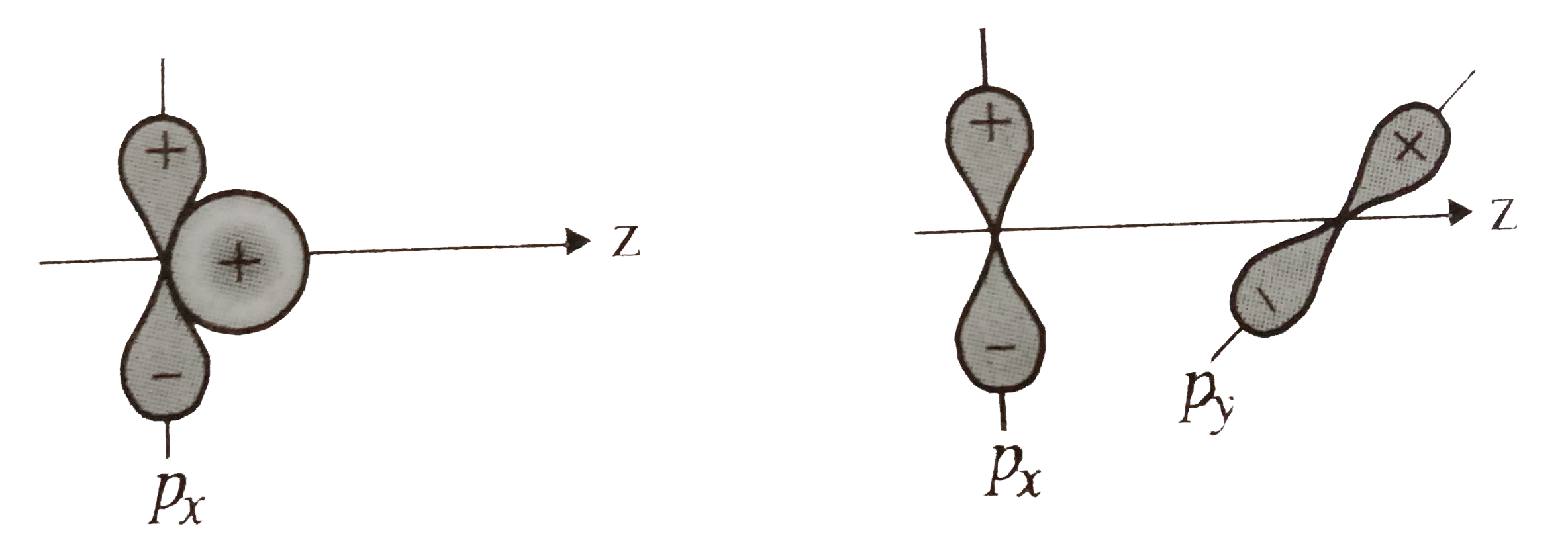Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Why does type of overlap given in the following figure not result in b...
Text Solution
|
- Why does type of overlap given in the following figure not result in b...
Text Solution
|
- Why does type of overlap given in the following figure not result in b...
Text Solution
|
- Which type of overlapping results in the formation of a pi bonds
Text Solution
|
- सह-संयोजक आबन्ध का निर्माण किस प्रकार के कक्षकों के अतिव्यापन द्वारा ...
Text Solution
|
- कथन : sigma-आबन्ध pi- आबन्ध की अपेक्षा अधिक प्रबल होता है । का...
Text Solution
|
- Which type of overlapping reusults in the formation of a pi bond?
Text Solution
|
- According to VBT the extent of overlapping depends upon types of orbit...
Text Solution
|
- According to VBT the extent of overlapping depends upon types of orbit...
Text Solution
|