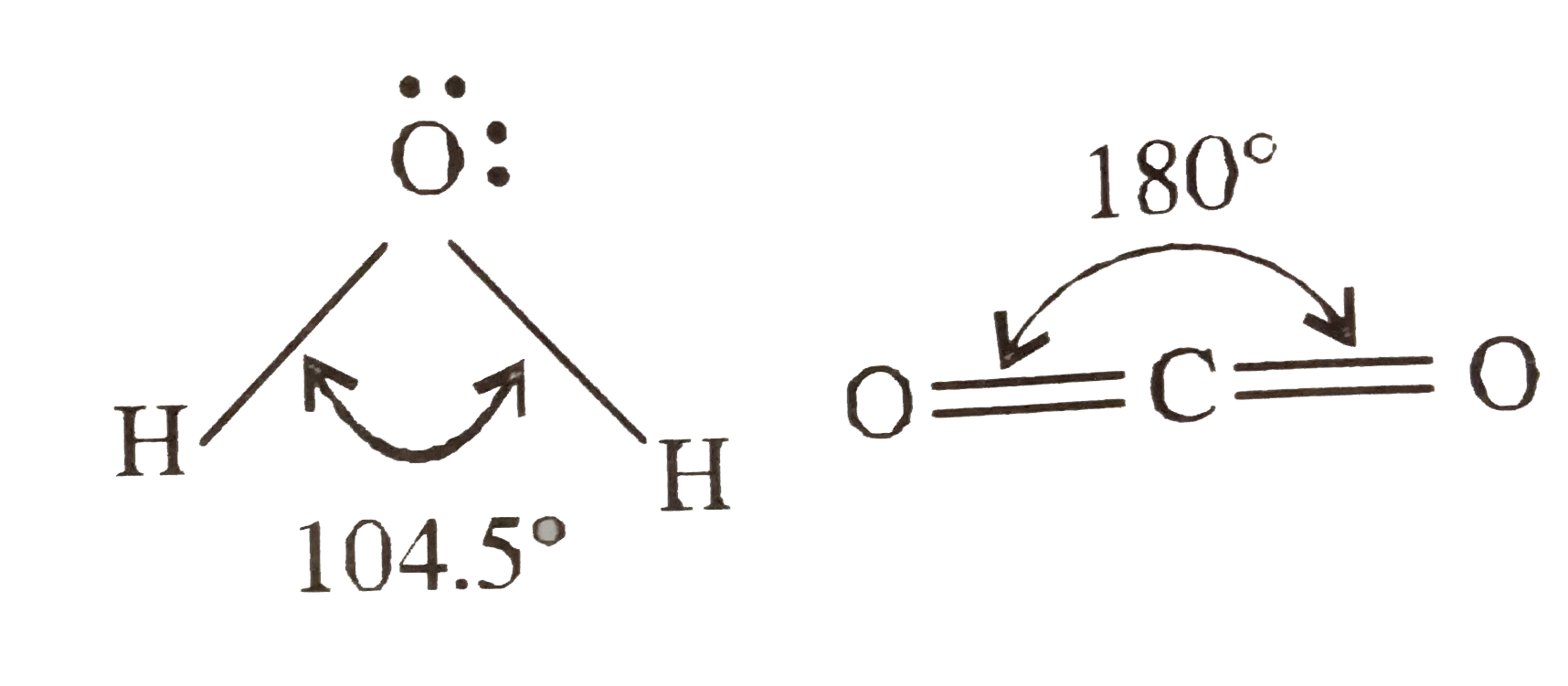Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Give reason for the following. a) Covalent bonds are directional bon...
Text Solution
|
- Ionic bonds are non-directional while covalent bonds are directional.
Text Solution
|
- How many covalent bonds are present in a molecule of carbon dioxide?
Text Solution
|
- Give reasons for the following : (i) Covalent bonds are directional...
Text Solution
|
- Give reason for the following. a) Covalent bonds are directional bonds...
Text Solution
|
- Give one example each of the following (i) A molecule containing a s...
Text Solution
|
- Give reasons for the following : (i) Covalent bonds are directional...
Text Solution
|
- Which of the following molecules have following : ionic bond, covale...
Text Solution
|
- Give reasons for the following : Covalent bonds are directional while ...
Text Solution
|