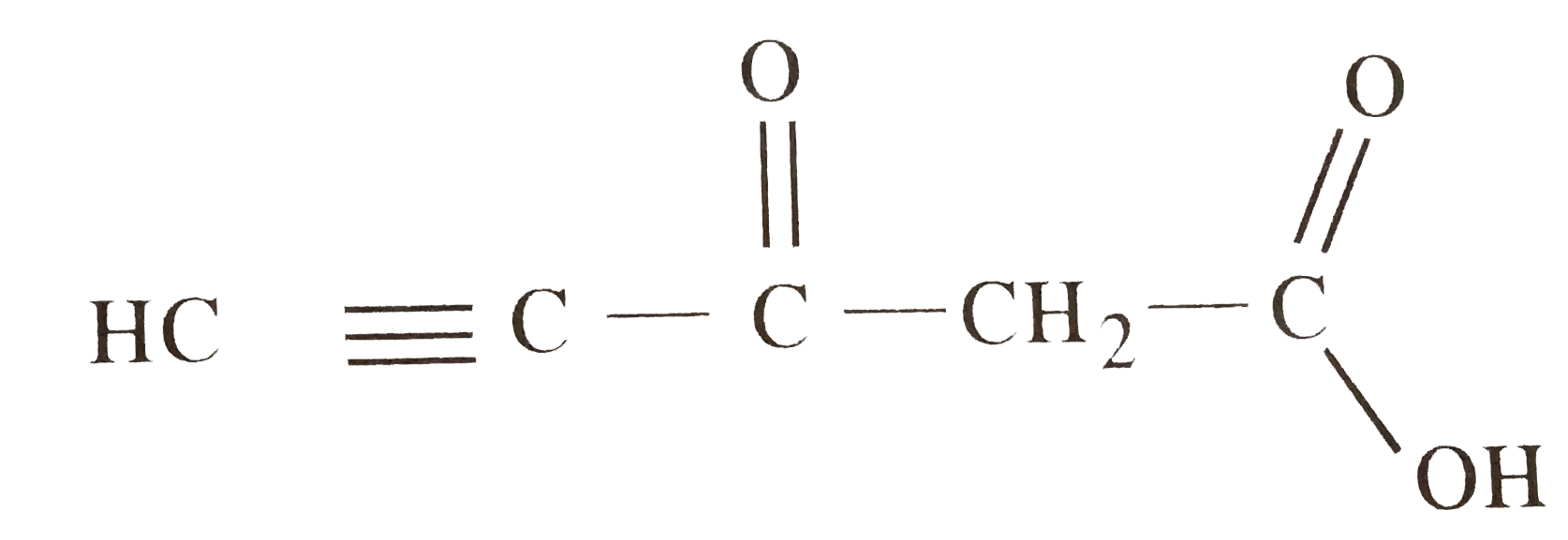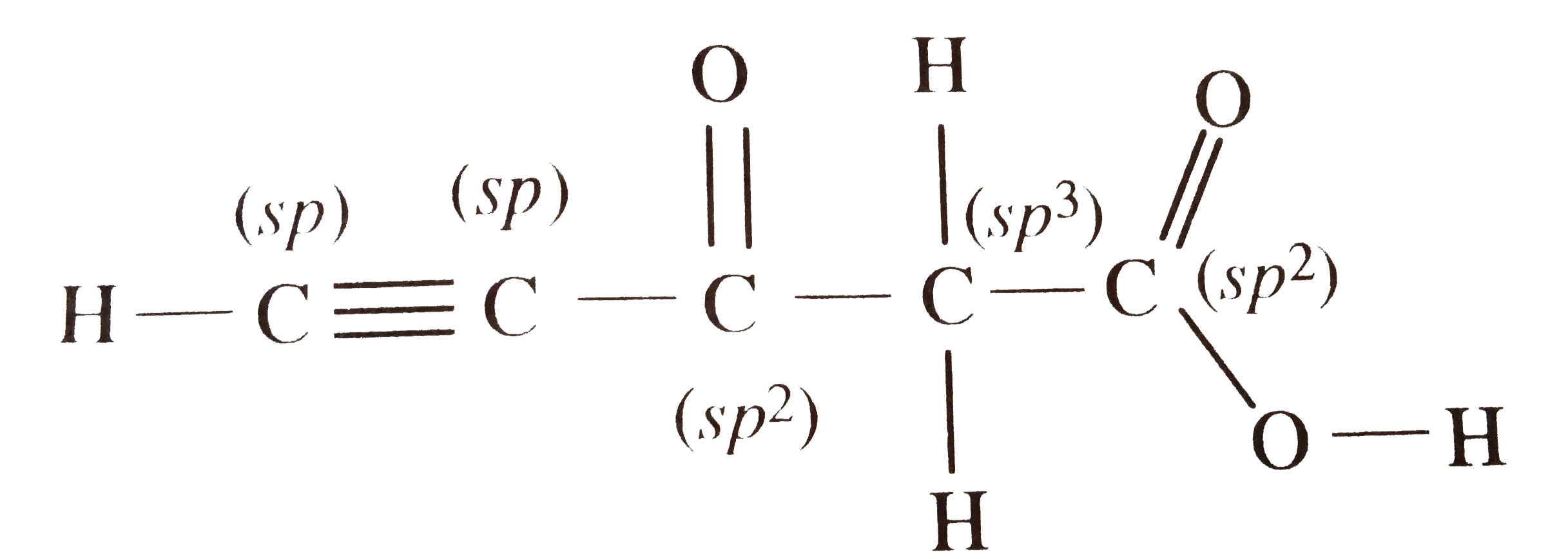Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Predict the hybridisation of each carbon in the molecule of organic co...
Text Solution
|
- The total number of sigma ( sigma ) and pi ( pi ) bonds in an ethylene...
Text Solution
|
- প্রদত্ত অনুগুলির মধ্যস্থিত sigma ও pi -বন্ধনগুলি নির্দেশ করো - C6H6
Text Solution
|
- Indicate the sigma and pi-bonds between the given molecules - C6H12
Text Solution
|
- প্রদত্ত অনুগুলির মধ্যস্থিত sigma ও pi -বন্ধনগুলি নির্দেশ করো - CH2Cl2
Text Solution
|
- প্রদত্ত অনুগুলির মধ্যস্থিত sigma ও pi -বন্ধনগুলি নির্দেশ করো - CH2=C=C...
Text Solution
|
- প্রদত্ত অনুগুলির মধ্যস্থিত sigma ও pi -বন্ধনগুলি নির্দেশ করো - CH3NO2
Text Solution
|
- প্রদত্ত অনুগুলির মধ্যস্থিত sigma ও pi -বন্ধনগুলি নির্দেশ করো - HCONHCH...
Text Solution
|
- Indicate the sigma and pi bonds in naphthalene and isobutane molecules...
Text Solution
|