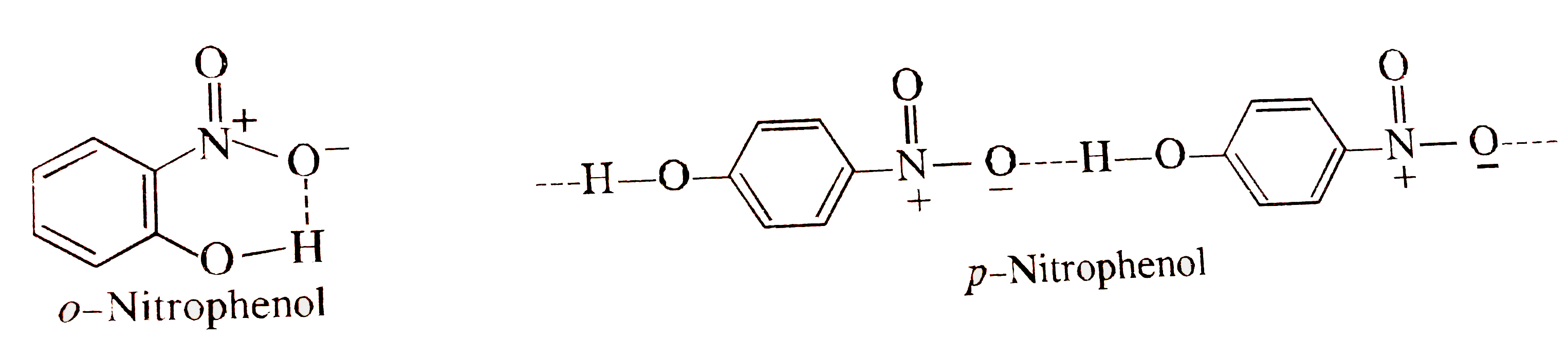Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Orthonitrophenol is steam volatile but paranitrophenol is not because ...
Text Solution
|
- Orthonitrophenol is steam volatile but paranitrophenol is not because ...
Text Solution
|
- Which of the following is non-volatile in steam ?
Text Solution
|
- Steam distillation is applied to those organic compound which are stea...
Text Solution
|
- The most steam volatile species is:
Text Solution
|
- Explain why si orthonitrophenol ?
Text Solution
|
- The most steam volatile compoud is
Text Solution
|
- Steam distillation is applied to those organic compounds which are ste...
Text Solution
|
- Assertion (A) o-and p-nitrophenol can be separated by steam distillati...
Text Solution
|