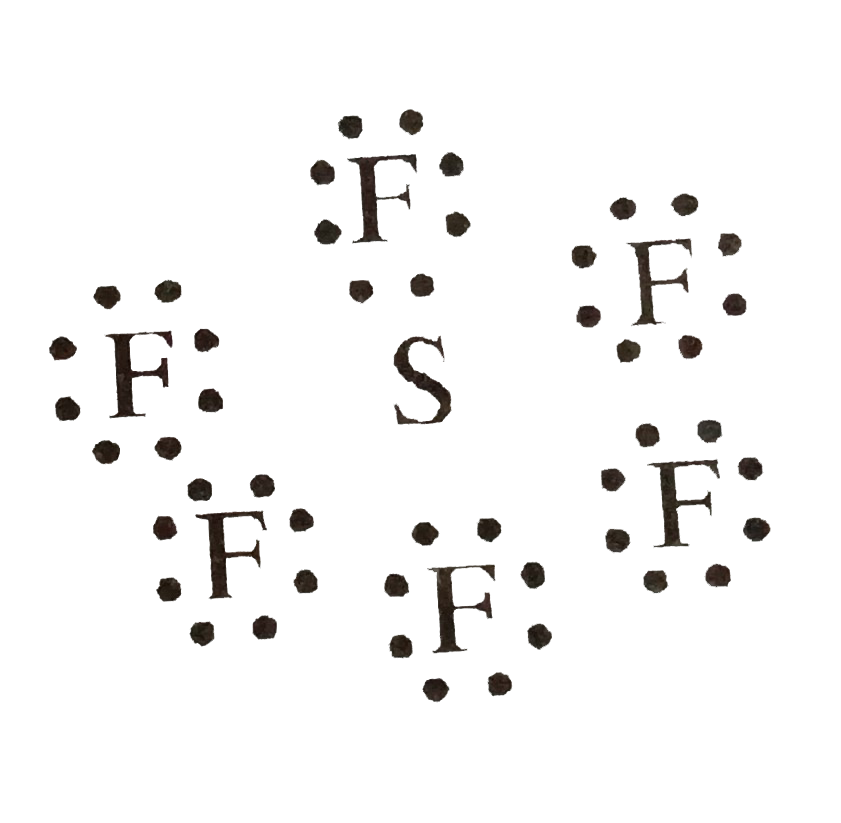Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- According to Octet Rule, each atom gains or loses electrons to complet...
Text Solution
|
- According to Octed Rule, each atom gains or loses electrons to complet...
Text Solution
|
- Assertion SF(6) is not a stable molecule. Reason . A stable molecule m...
Text Solution
|
- Beryllium has 2 electrons in its outer shell. so according to octet ru...
Text Solution
|
- What is producer gas ?
Text Solution
|
- In methane molecules , central carbon atom has electrons in its ...
Text Solution
|
- The molecule in which central atom is associated with more than 8 elec...
Text Solution
|
- Identify the molecule which has more than 8 electrons in outermost orb...
Text Solution
|
- The number of electrons in the valence shell of the central atom of a ...
Text Solution
|