Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
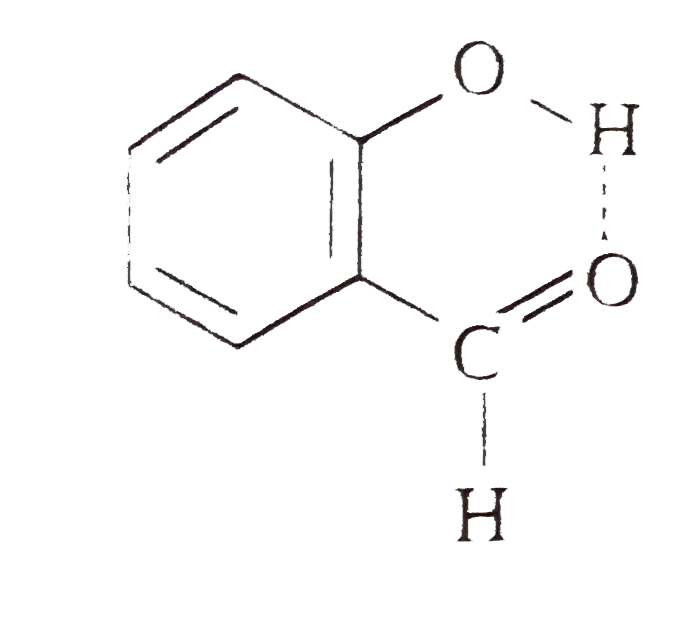
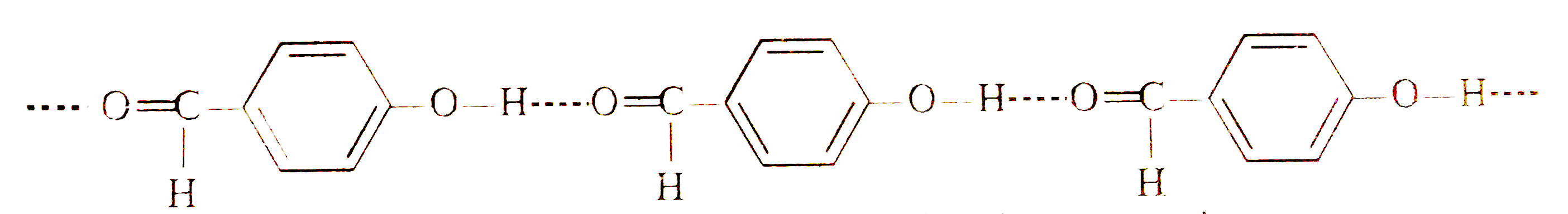
- o-Hydroxybenzaldehyde is a liquid at room temperature while p-hydroxyb...
Text Solution
|
- p-Hydroxybenzaldehyde does not undergo Cannizzaro reaction. Explain.
Text Solution
|
- Explain, why o -hydroxybenzaldehyde is a liquid at room temperature wh...
Text Solution
|
- Explain why is o-hydroxybenzaldehyde a liquis at room temperaturre whi...
Text Solution
|
- Explain why o-hydroxybenzaldehyde is liquid at room temperature while ...
Text Solution
|
- Explain, why o-hydroxybenzaldehyde is a liquid at room temperature whi...
Text Solution
|
- At normal temperature o-hydroxybenzaldehyde is a liquid but p-hydroxyb...
Text Solution
|
- Suggest a method to separate a mixture of o-hydroxy-benzaldehyde and p...
Text Solution
|
- व्याख्या कीजिए कि कमरे के ताप पर, o-हाइड्रॉक्सी बेंजैल्डिहाइड द्रव है ...
Text Solution
|