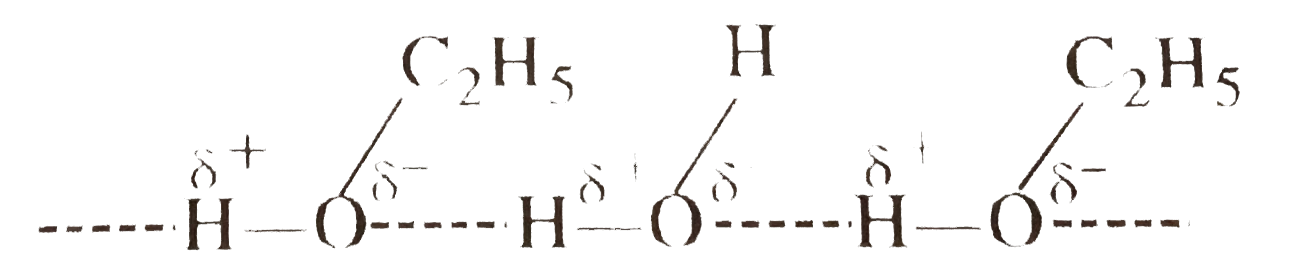Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- State with reasons : (i) Which is more acidic : anhydrous HCl or aqu...
Text Solution
|
- Which alcohol is most reactive towards HCl in the presence of anhydrou...
Text Solution
|
- State with reasons : (i) Which is more acidic : anhydrous HCl or aqueo...
Text Solution
|
- Assertion :-HCl is more polar than HF. Reason :- Cl has more E.N. than...
Text Solution
|
- Which gas, out of O(2) and CO(2) is more soluble ?
Text Solution
|
- Which is more polar and why, CO(2) or N(2)O?
Text Solution
|
- Which of the following alcohol is more soluble in water ?
Text Solution
|
- Which out of anhydrous and hydrous AlCl3 is more soluble in ether and ...
Text Solution
|
- Which is more polar and why, CO(2) or N(2)O?
Text Solution
|