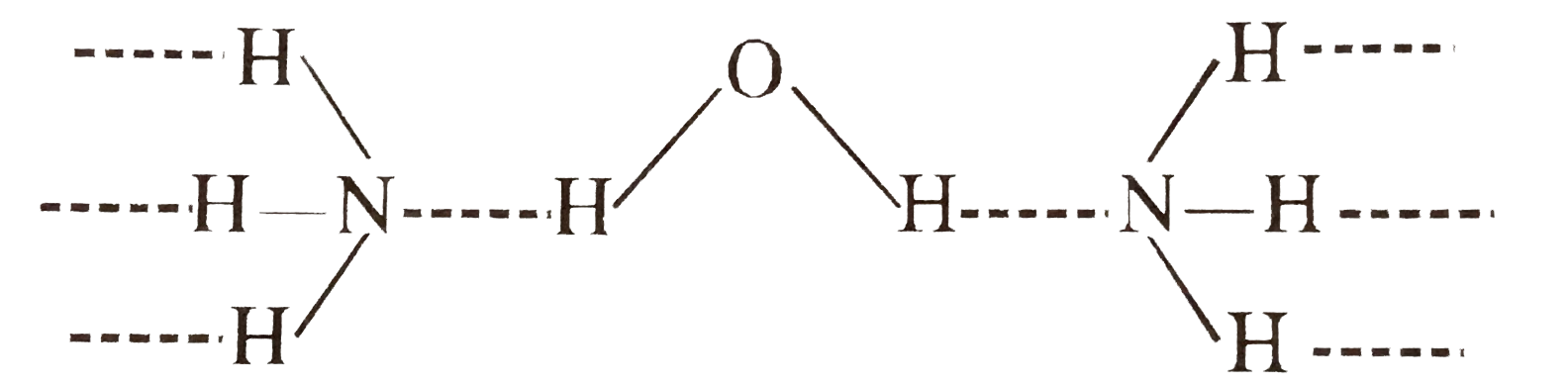
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assign reason for the following : (i) Ammonia is soluble in water wh...
Text Solution
|
- Assign a reason for each of the following statements : (i) Ammonia is ...
Text Solution
|
- Assign reason for the following : (i) Ammonis is soluble in water whil...
Text Solution
|
- Ammonia is soluble in water but phosphine is insoluble because
Text Solution
|
- Assign a reason for each of the following statements : (i) Ammonia is ...
Text Solution
|
- CuCl जल में अविलेय है जबकि CuCl(2) जल में विलेय है क्यों ?
Text Solution
|
- B(2) is paramagnetic but C(2) is not. Explain.
Text Solution
|
- Assign reason for the following, NH(3) is freely soluble in water whil...
Text Solution
|
- Assign reason for the following, B(2) is paramagnetic while C(2) is no...
Text Solution
|