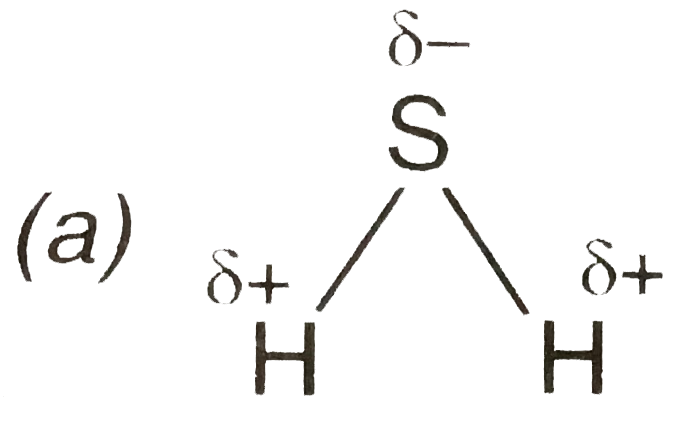A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The geometry of H2 S and its dipole moment are :
Text Solution
|
- Dipole Moment And Its Applications
Text Solution
|
- The geometry of H2 S and its moment are :
Text Solution
|
- Assertion : The dipole moment helps to predict whether a molecule is ...
Text Solution
|
- H(2)S की ज्यामिति और इसका द्विध्रुव है :
Text Solution
|
- कथन : BF(3) अणु का परिणामी द्विध्रुव आघूर्ण शून्य होता है । क...
Text Solution
|
- H(2)S की ज्यामिति तथा इस द्विध्रुव आघूर्ण है-
Text Solution
|
- The geometry of H(2)S and its dipole moment is-
Text Solution
|
- H(2)S की ज्यामिति (geometry) तथा इसका द्विध्रुव आघूर्ण क्रमशः है
Text Solution
|