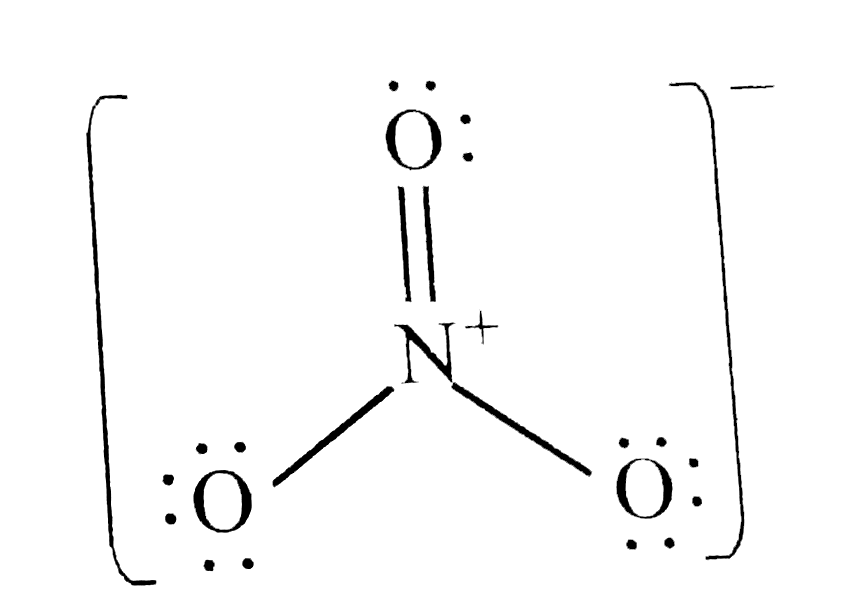
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In NO(3)^(-) ion, the number of bond pair and lone pair of electrons o...
Text Solution
|
- In NO3^- ion, the number of bond pair and lone pair of electrons no N-...
Text Solution
|
- In NO(3)^(-) ion, the number of bond pair and lone pair of electrons o...
Text Solution
|
- In NO(3)^(-)ion, the number of bond pairs and lone pairs of electrons ...
Text Solution
|
- In NO(3)^(-) ion, the number of bond pairs and lone pairs of electrons...
Text Solution
|
- In NO3^+ ion, number of bond pairs and lone pairs of electrons are res...
Text Solution
|
- In NO(3)^(-) ion, the number of bond pairs and lone pairs of electrons...
Text Solution
|
- In NO(3)^(-) ion, the number of bond pair and lone pair of electrons o...
Text Solution
|
- In NO(3)^(-) ion, the number of bond pairs and lone pairs of electrons...
Text Solution
|