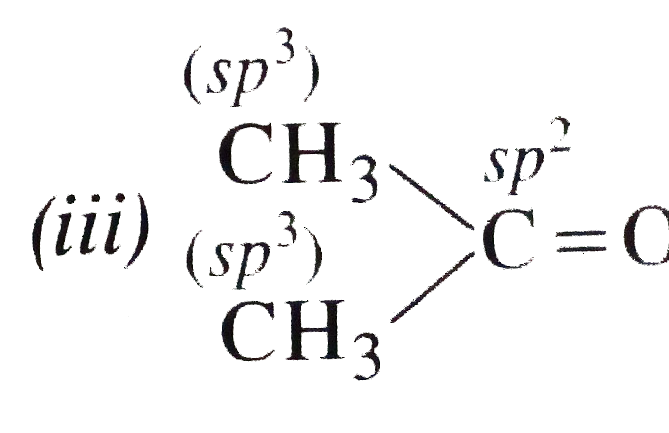Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- What are hybridisation states of each carbon atom in the following com...
Text Solution
|
- Given the hybridistaiton state of each carbon in the following compoun...
Text Solution
|
- What are the hydridised states of carbon atoms in the following compou...
Text Solution
|
- What are hybridisation states of each carbon atom in the following c...
Text Solution
|
- निम्नलिखित यौगिकों में प्रत्येक कार्बन की संकरण अवस्था बताइए- CH(2)=...
Text Solution
|
- Name the following halides according to IUPAC system and classify them...
Text Solution
|
- What are hybridisation states of each carbon atom in the following com...
Text Solution
|
- What are hybridistion states of each C - atom in the compounds: (1) ...
Text Solution
|
- What are hybridisation states of each carbon atom in the following com...
Text Solution
|