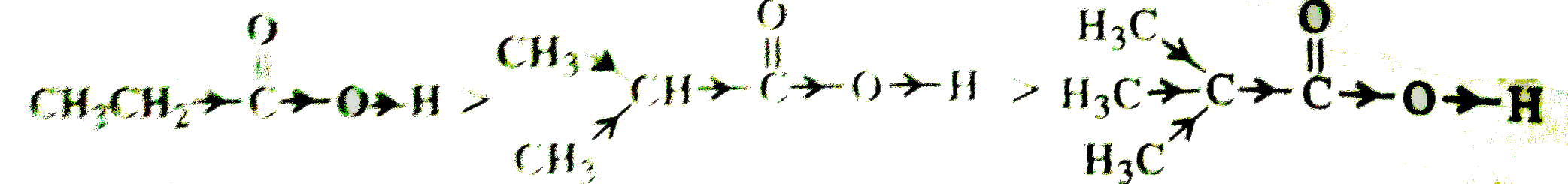
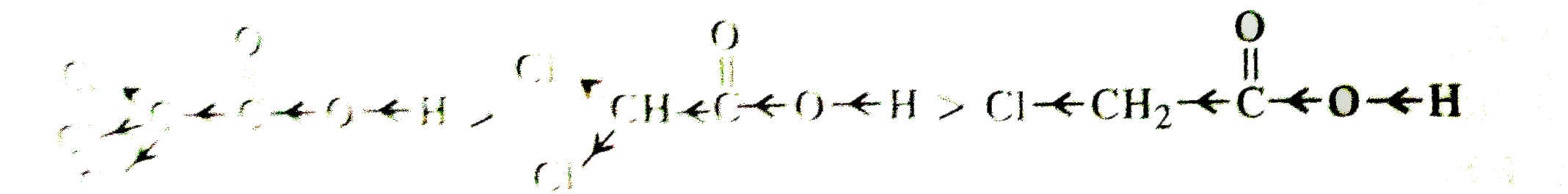Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain the terms Inductive and Electromeric effects. Which electron d...
Text Solution
|
- Arrange the following as stated : 'Increasing order of acidic streng...
Text Solution
|
- Explain the terms Inductive and Electromeric effects. Which electron d...
Text Solution
|
- Arrange the following in order of increasing acidic strength (i) HCOOH...
Text Solution
|
- Arrange the following carboxylic acids in the decreasing order of thei...
Text Solution
|
- Explain the terms Inductive and Electromeric effects. Which electron d...
Text Solution
|
- निम्नलिखित कार्बोक्सिलिक अम्लों की अम्लता का सही कर्म कौन-सा इलेक्ट्रॉ...
Text Solution
|
- Which of the following is the correct orderr of acidity of carboxylic ...
Text Solution
|
- Arrange the following in increasing order of acid strength: ClCH(2)COO...
Text Solution
|