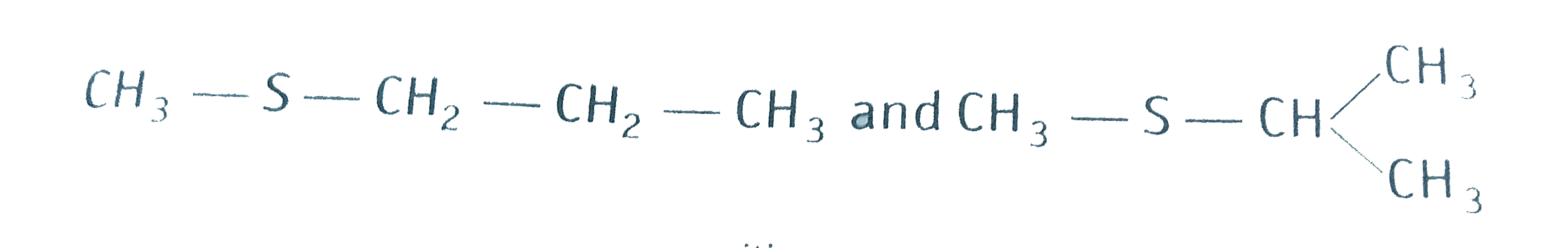Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compounds with same molecular formula but differing in their structure...
Text Solution
|
- Compounds with same molecular formula but differing in their structure...
Text Solution
|
- Compound with same molecular formula but different structure formula a...
Text Solution
|
- Molecular formula of a hydrocarbon is C5H(10) .What type of isomerisms...
Text Solution
|
- Compounds having the same molecular formula but differing in th...
Text Solution
|
- Complex compounds which have same molecular formula but have different...
Text Solution
|
- Complex compounds which have same molecular formula but have different...
Text Solution
|
- Compounds with same molecular formula and different structural formula...
Text Solution
|
- Molecules having same molecular formula. But differing in structure (o...
Text Solution
|