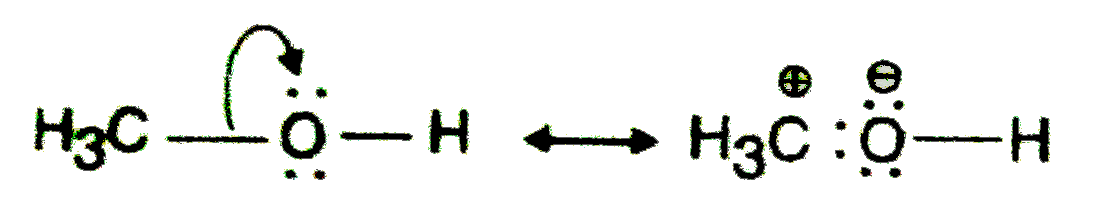
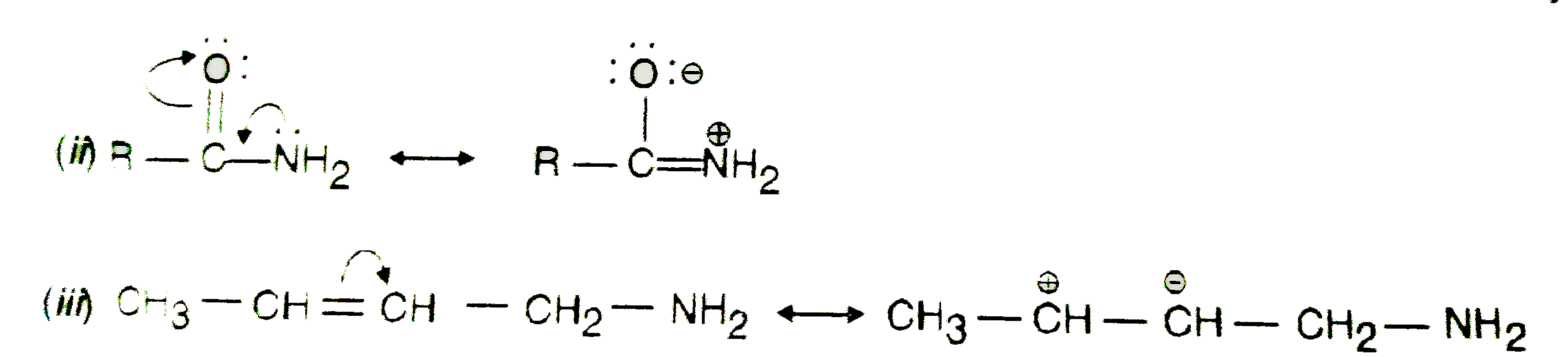Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds will not exist as resonance hybrid. G...
Text Solution
|
- The IUPAC name of the compound CH(3)CH=CHCH(2)underset(NH(2))underset(...
Text Solution
|
- Which of the following compounds will not exist as resonance hybrid. G...
Text Solution
|
- Explain the formation of the products in the following reaction. CH(...
Text Solution
|
- Which of the following compounds will not exist as resonance hybrid. G...
Text Solution
|
- निम्नलिखित अभिक्रिया मे उत्पाद के निर्माण की व्याख्या कीजिए - CH(3)C...
Text Solution
|
- compounds X is treated with NH(2) OH and followed by reduction gives :...
Text Solution
|
- Compound X is the treated with NH(2)OH and by reduction gives . (CH(3)...
Text Solution
|
- Write the IUPAC names of the compound CH(2)=CHCH(2)CH(2)C-=CH&CH(3)CH=...
Text Solution
|