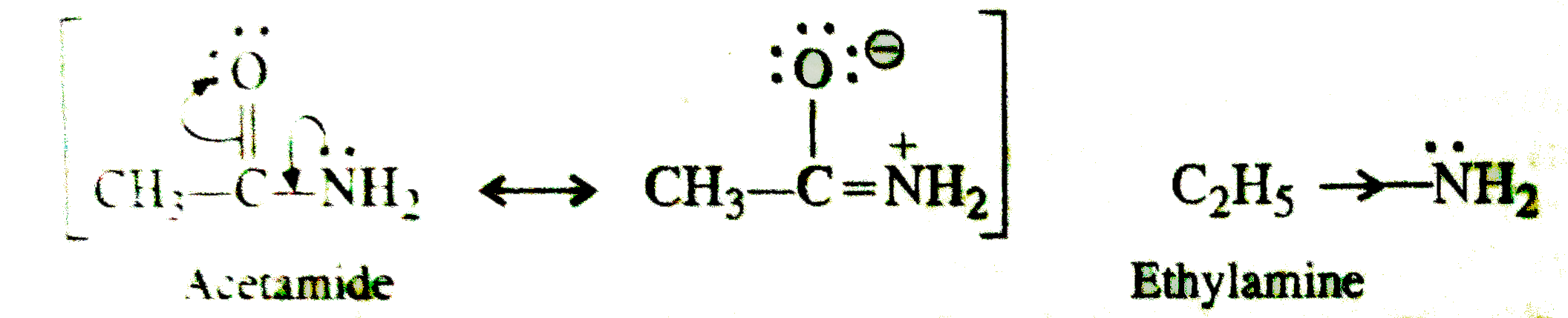Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Ethylamine is a stronger base than acetamide. Assign reason.
Text Solution
|
- Ethylamine is a stronger base than acetamide. Assign reason.
Text Solution
|
- Out of ethylamine and acetamide which is a stronger base and why?
Text Solution
|
- Boron tribromide is a stronger acid than boron trifuoride. Assign reas...
Text Solution
|
- Acetamide and ethylamine are distmgmshed by reacting with:
Text Solution
|
- Give Reasons: Methylamine is a stronger base than ammonia.
Text Solution
|
- Assign a reason for the following statements: Alkylamines are stronger...
Text Solution
|
- Explain the ethylamine is stronger than ammonia?
Text Solution
|
- एल्केनेमीन अमोनिया से प्रबल क्षारक है। कारण दीजिये।
Text Solution
|