Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
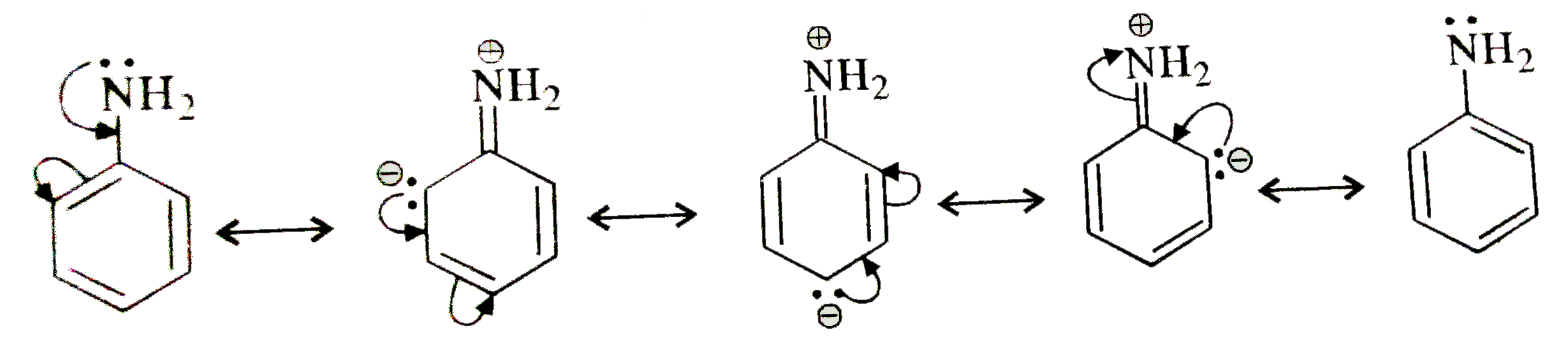
- Cyclohexylamine is more basic than aniline. Explain
Text Solution
|
- Aniline is more basic than
Text Solution
|
- Cyclohexylamine is more basic than aniline. Explain
Text Solution
|
- The basicity of aniline is less than that of cyclohexylamine. This is ...
Text Solution
|
- एथिल ऐमीन, ऐनिलीन से अधिक क्षारकीय है, समझाइए।
Text Solution
|
- Cyclohexylamine is stronger base than aniline because
Text Solution
|
- Give reason: Aniline is a weaker base than cyclohexylamine.
Text Solution
|
- The basicity of aniline is less than that of cyclohexylamine. This is ...
Text Solution
|
- Aniline is less/more basic than ethylamine.
Text Solution
|