Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
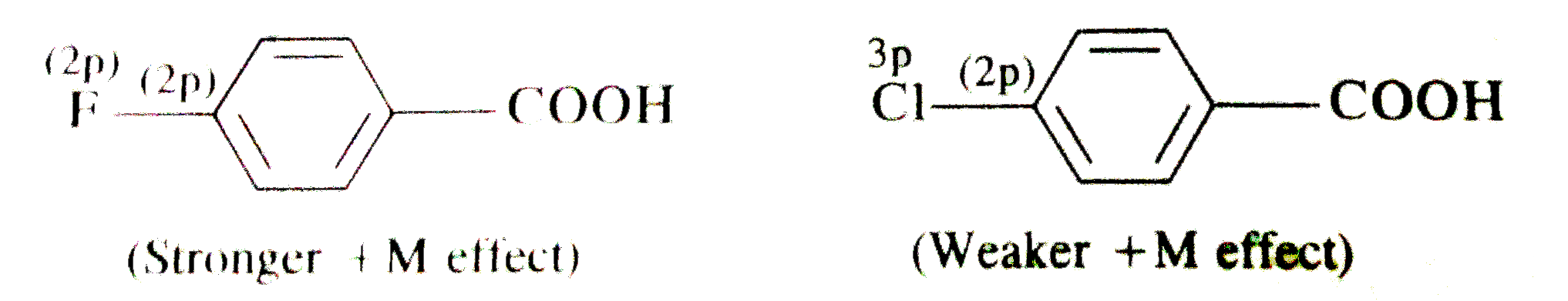
- Fluorine is more electronegative than chlorine but p-fluorobenzoic aci...
Text Solution
|
- Despite the fact than fluorine is more electronegative than iodine, ye...
Text Solution
|
- m-Hydroxybenzoic acid is a stronger acid than benzoic acid while p-hyd...
Text Solution
|
- Fluorine is more electronegative than chlorine but p-fluorobenzoic aci...
Text Solution
|
- Assertion : Although fluorine is more electronegative than chlorine, p...
Text Solution
|
- Fluorine is more electronegative than chlorine but p-fluorobenzoic aci...
Text Solution
|
- Assertion: p-Chlorobenzoic acid is stronger than benzoic acid. Reason:...
Text Solution
|
- Assertion : Although fluorine is more electronegative than chlorine, p...
Text Solution
|
- BF3 is a weaker Lewis acid than BCl3 , even though F is more electrone...
Text Solution
|