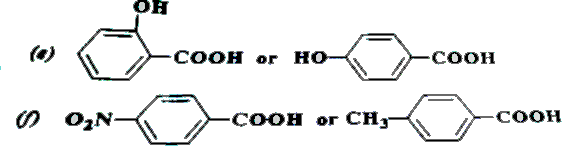
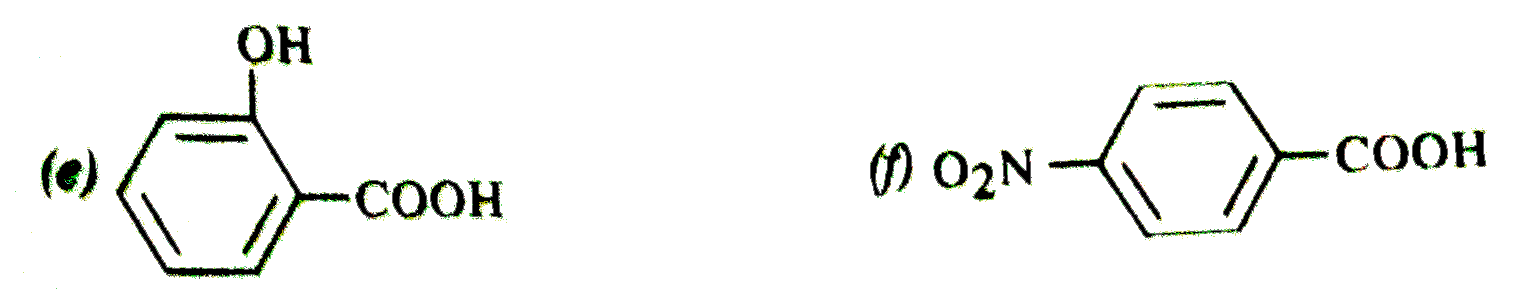Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which out of each pair is expected to be a stornger acid ? (a) CH(3)...
Text Solution
|
- Which out of each pair is expected to be a stornger acid ? (a) CH(3)...
Text Solution
|
- How many of the carboxylic acid reduce tollen's reagent ? CH(3)COOH,...
Text Solution
|
- Arrange the following in order of increasing acid strength: (a) NH(3...
Text Solution
|
- Which is decreasing order of acidity in, HCOOH(I), CH(3)COOH(II), CH...
Text Solution
|
- For the following acids the rate of decarboxylation on heating would b...
Text Solution
|
- निम्नलिखित अम्लों को उनकी बढ़ी हुई प्रबलता के कर्म में लिखिए- C(6)H(5...
Text Solution
|
- The decreasing acidic strength of a few of the acids HCOOH, CH(3)COOH...
Text Solution
|
- (i) F(3)C-COOH, (ii) CH(3)COOH , (iii) C(6)H(5)COOH, (iv) CH(3)CH(2)CO...
Text Solution
|