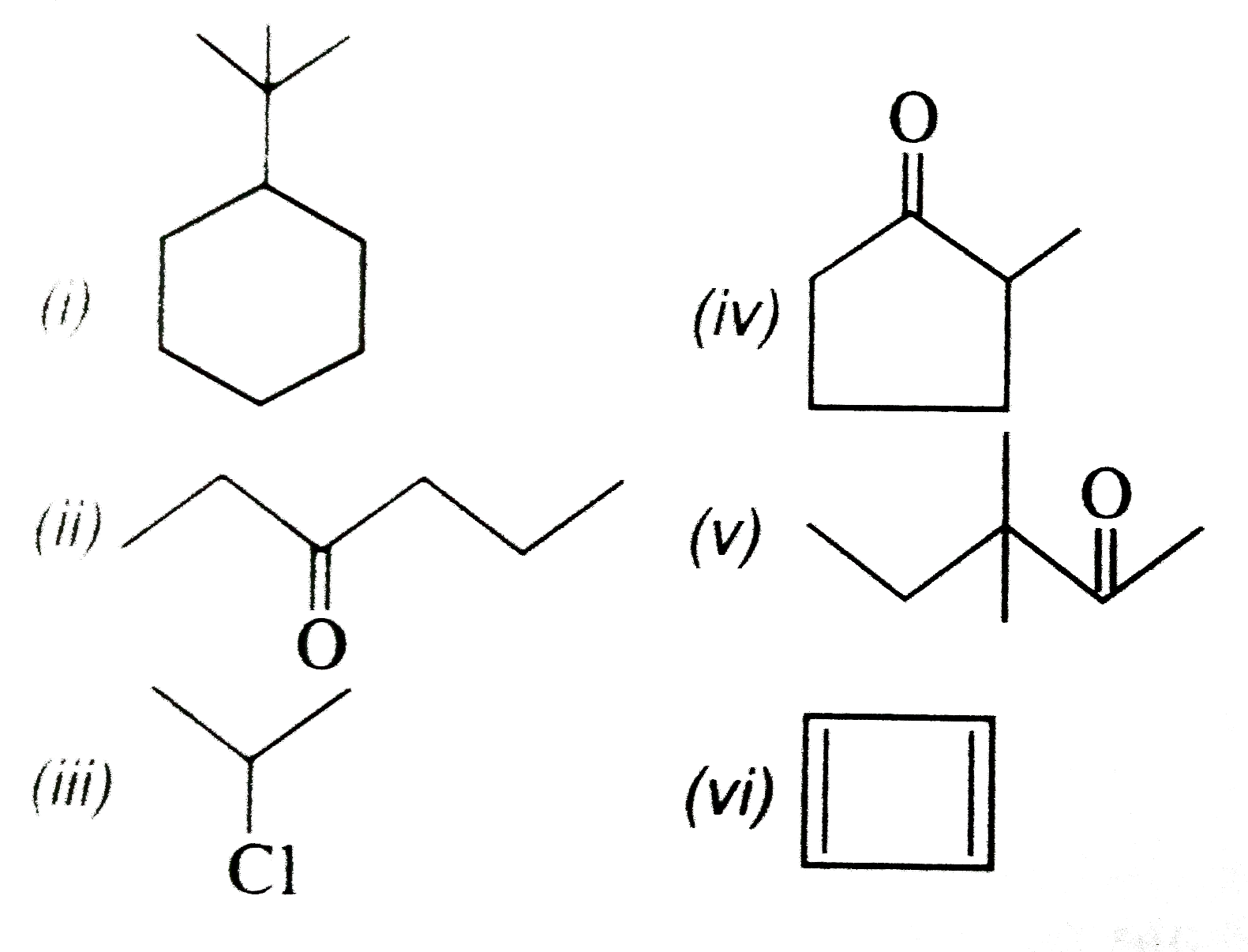Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write bond line notation formulae for (i) Tert-butylcyclohexane (i...
Text Solution
|
- Write the structure of : (i) (E) penta-1,3-diene " " (ii) (2Z,4E)yhexa...
Text Solution
|
- Write bond line formulas for : (i) Isopropyl alcohol (ii) 2, 3-Dimethy...
Text Solution
|
- Write bond line notation formulae for (i) Tert-butylcyclohexane (i...
Text Solution
|
- Draw the structure of the following : (i) 3-Methylbutanal , (ii) p-M...
Text Solution
|
- Find the values of :- (i)2^(4) (ii)4^(3) (iii)3^(2) (iv)5^(3) ...
Text Solution
|
- Write the condensed formulae for each of the following compounds : (i)...
Text Solution
|
- Write bond line formulas for : (i) Isopropyl alcohol (ii) 2, 3-Dimet...
Text Solution
|
- Write bond line formulas for : (i) Isopropyl alcohol (ii) 2, 3-Dimet...
Text Solution
|