Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
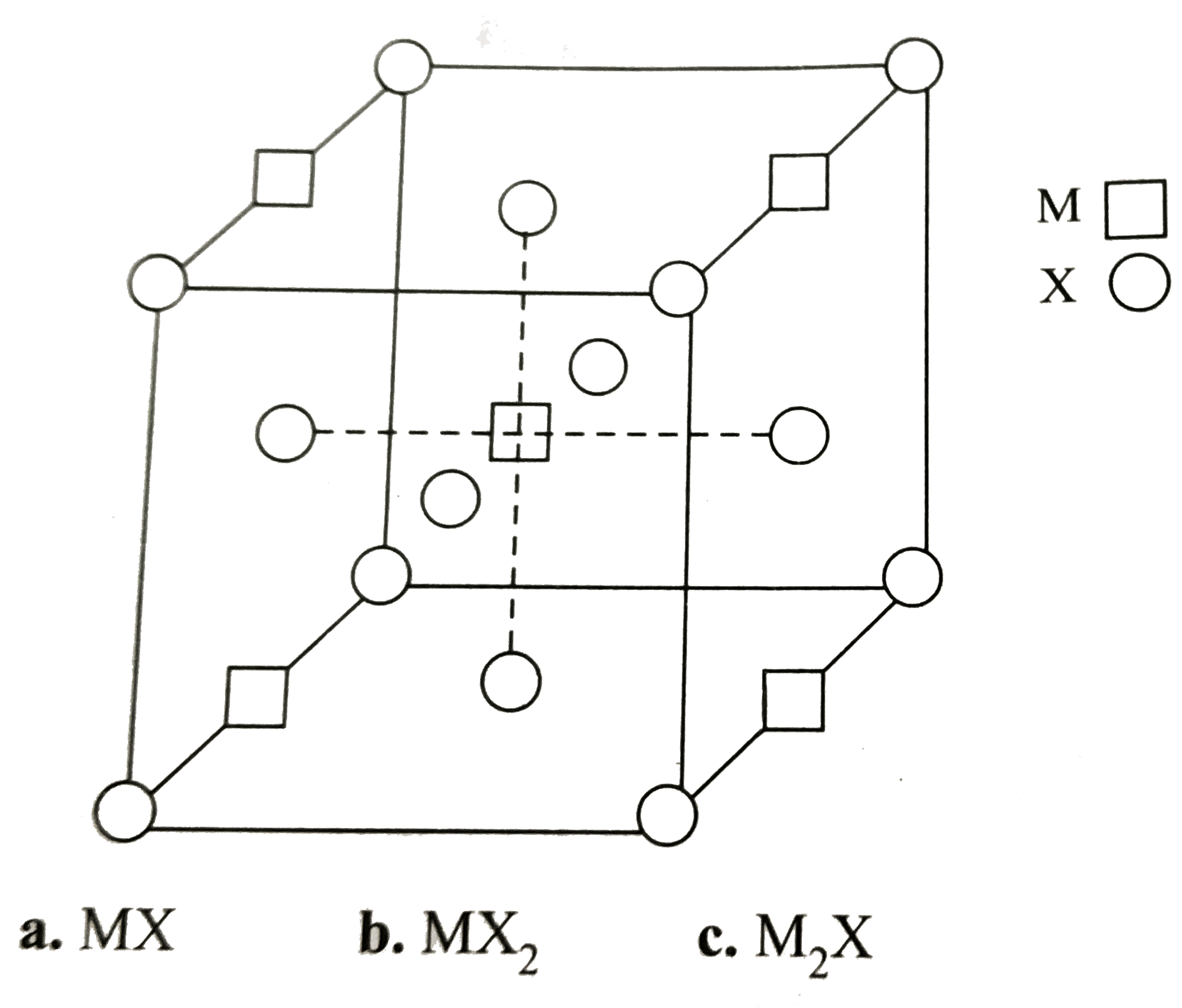
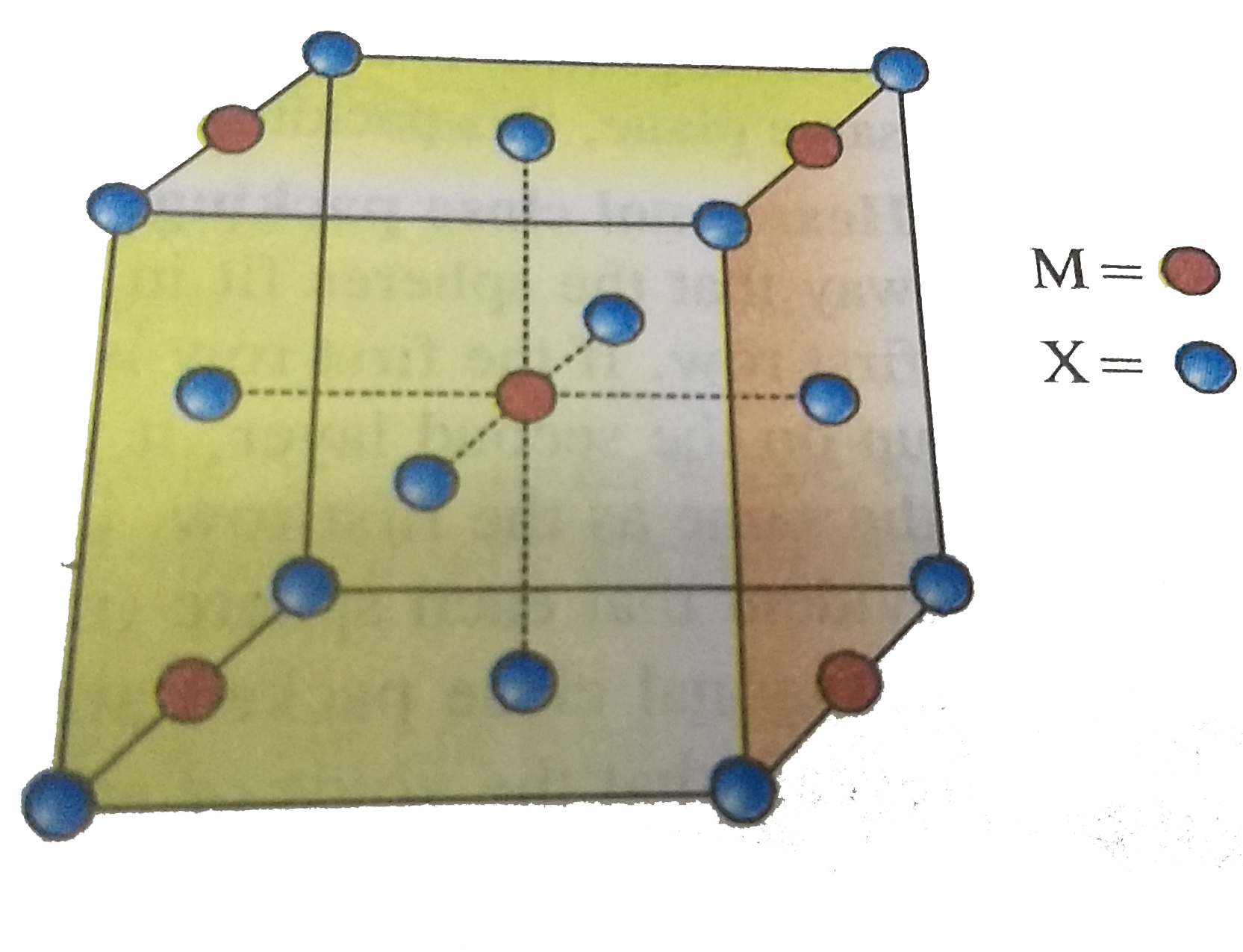
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- In the cubic close close packing, the unit cell has....
Text Solution
|
- A compound M(p)X(q) has cubic close packing (ccp) arrangement of X. It...
Text Solution
|
- In the cubic close close packing, the unit cell has....
Text Solution
|
- In the cubic close close packing, the unit cell has....
Text Solution
|
- A compound Mp Xq has cubic close packing (ccp) arrangement of X. Its u...
Text Solution
|
- एक यौगिक M(p) X(q) में X की घनीय संवृत संकुलन (ccp) व्यवस्था है । इसकी...
Text Solution
|
- What is the unit cell in a cubic close-packed (ccp) structure of parti...
Text Solution
|
- In the cubic close packing, the unit cell has-
Text Solution
|