A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
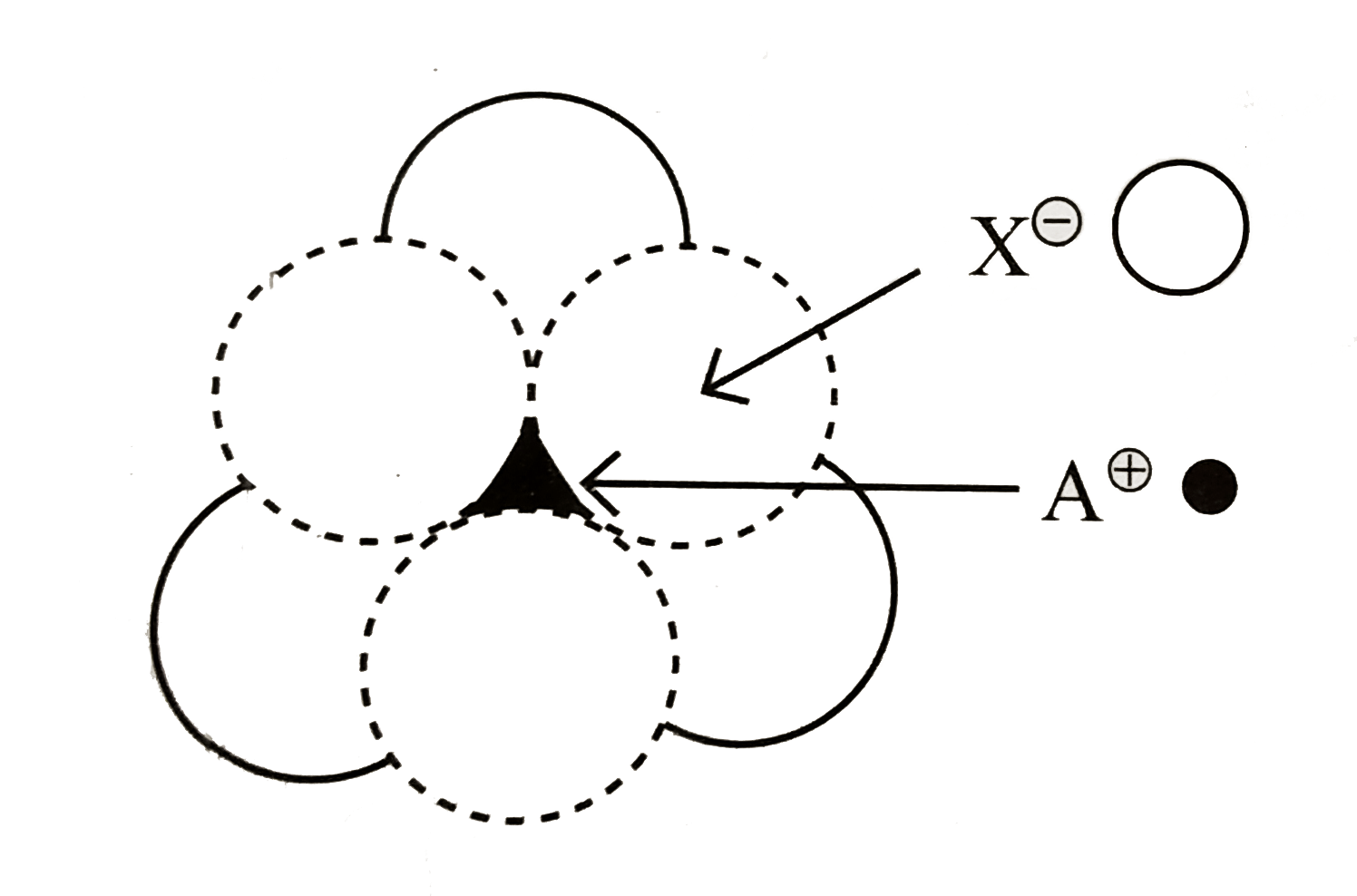
- The arrangement of X^(ɵ) ions around A^(o+) ion in solid AX is given i...
Text Solution
|
- Assertion (A) : In sodium chloride crystal, Na^(o+) ions occupy OVs wh...
Text Solution
|
- A solid A^(o+)B^(ɵ) has NaCl-type close-packed structure. If the radiu...
Text Solution
|
- Among the ions Cl^(ɵ), S^(2-) and Na^(o+) , the largest ion is "" .
Text Solution
|
- The arrangement of X^(-) ions around A^(+) in solids AX is given in th...
Text Solution
|
- The arrangement of X^(-)ions around A^(-)ion is solid AX is given in t...
Text Solution
|
- ठोस AX में A^(+) आयन के चारो और X^(-) आयन की व्यवस्था है, जैसा की चित्...
Text Solution
|
- The arrangement of X^(-) ions around A^(+) ion in solid AX is given in...
Text Solution
|
- एक ठोस Ax में A^+ आयन पर x^- आयनों की व्यवस्था चित्र में दी गई है। यदि...
Text Solution
|