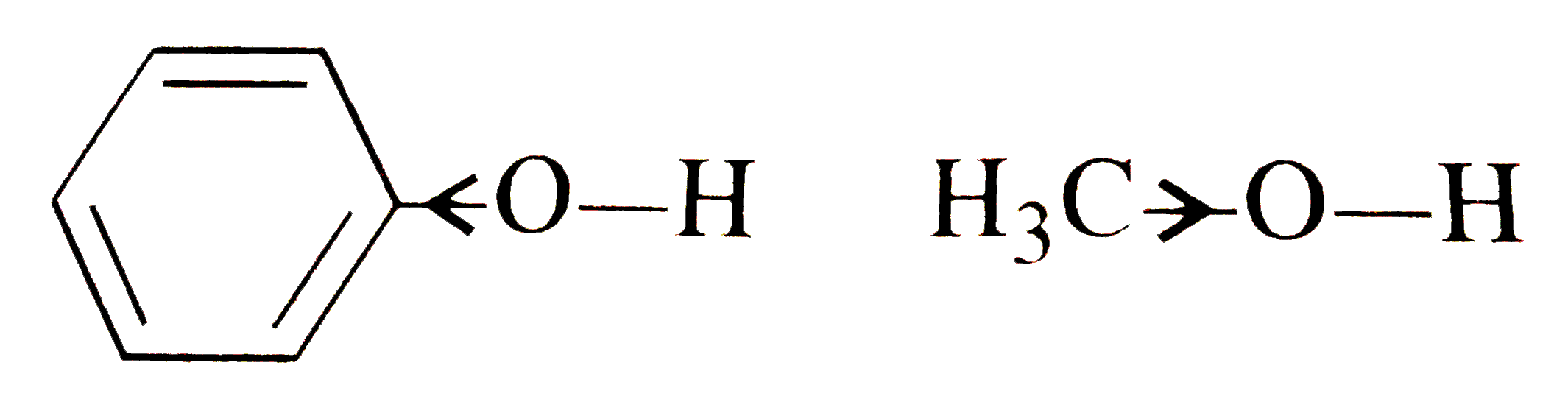Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Dipole moment of phenol is smaller than that of methanol. Why ?
Text Solution
|
- Dipole moment of phenol is smaller than that of methanol. Why ?
Text Solution
|
- CH3OH का द्विध्रुव आघूर्ण फीनॉल से अधिक होता है, क्यों?
Text Solution
|
- Dipole moment of phenol is smaller than that of methanol. Why ?
Text Solution
|
- Account for the following, (i) Phenol has a smaller dipole moment than...
Text Solution
|
- Account for the following : (i) Phenol has a smaller dipole moment t...
Text Solution
|
- फीनॉलों का द्विध्रुव आघूर्ण ऐल्कोहॉल से कम क्यों होता है?
Text Solution
|
- Why has phenol smaller dipole moment than methanol ?
Text Solution
|
- Phenol has smaller/larger dipole moment than methanol
Text Solution
|