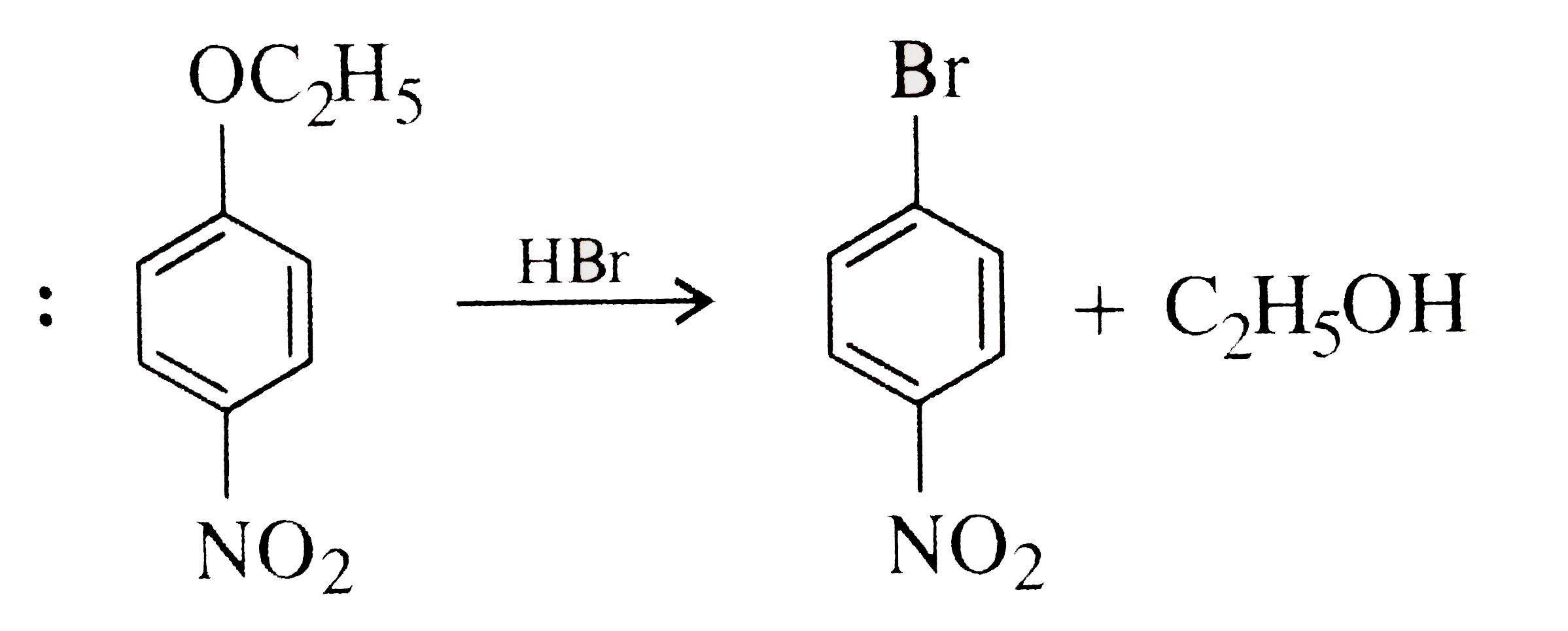A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion : Reason : Alkyl arly ethers on reaction with halogen aci...
Text Solution
|
- Assertion (A): Alkyl aryl ethers on reaction with HI give alkyl iodide...
Text Solution
|
- The reaction of an aromatic halogen compound with an alkyl halides in ...
Text Solution
|
- Assertion: Reason: due to formation of highly stable carbocation.
Text Solution
|
- Assertion (A) Gabriel phthalimide reaction can be used to prepare aryl...
Text Solution
|
- Assertion : When Alkyl aryl ethers react with excess of hydrogen halid...
Text Solution
|
- Aryl halides are less reactive than alkyl halide in mucleophilic subst...
Text Solution
|
- The reaction of an aromatic halogen compound with an alkyl halide in p...
Text Solution
|
- Ethers react with the formation of ethyl halide with halogen acids ...
Text Solution
|