Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- 0.037 g of alcohol R - OH was added to CH(3)MgBr and the gas ev...
Text Solution
|
- 0.037gm of an alcohol was added to CH(3)Mgl and the gas evolved measur...
Text Solution
|
- 0.037 g of an alcohol, ROH was added to CH(3)MgI and the gas evolved m...
Text Solution
|
- The treatment of CH(3)OH "with" CH(3)Mgl releases 1.04mL of a gas at S...
Text Solution
|
- The gas evolved on heating CH(3)MgBr in methanol is :
Text Solution
|
- 0.037g of an alcohol, R-OH was added to CH(3)MgBr and the gas evolved ...
Text Solution
|
- 0.037g of an alcohol, R-OH was added to CH(3)MgBr and the gas evolved ...
Text Solution
|
- A sample of 6.75mg of unknown alcohol is added to CH(3)MgBr when 2.52m...
Text Solution
|
- An alcohol on oxidation is found to give CH(3)COOH and CH(3)CH(OH)CH(2...
Text Solution
|
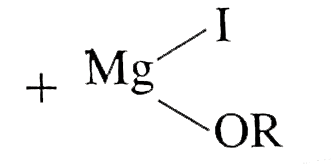 ,(0.44g,11cm^(3),):}`
,(0.44g,11cm^(3),):}` 