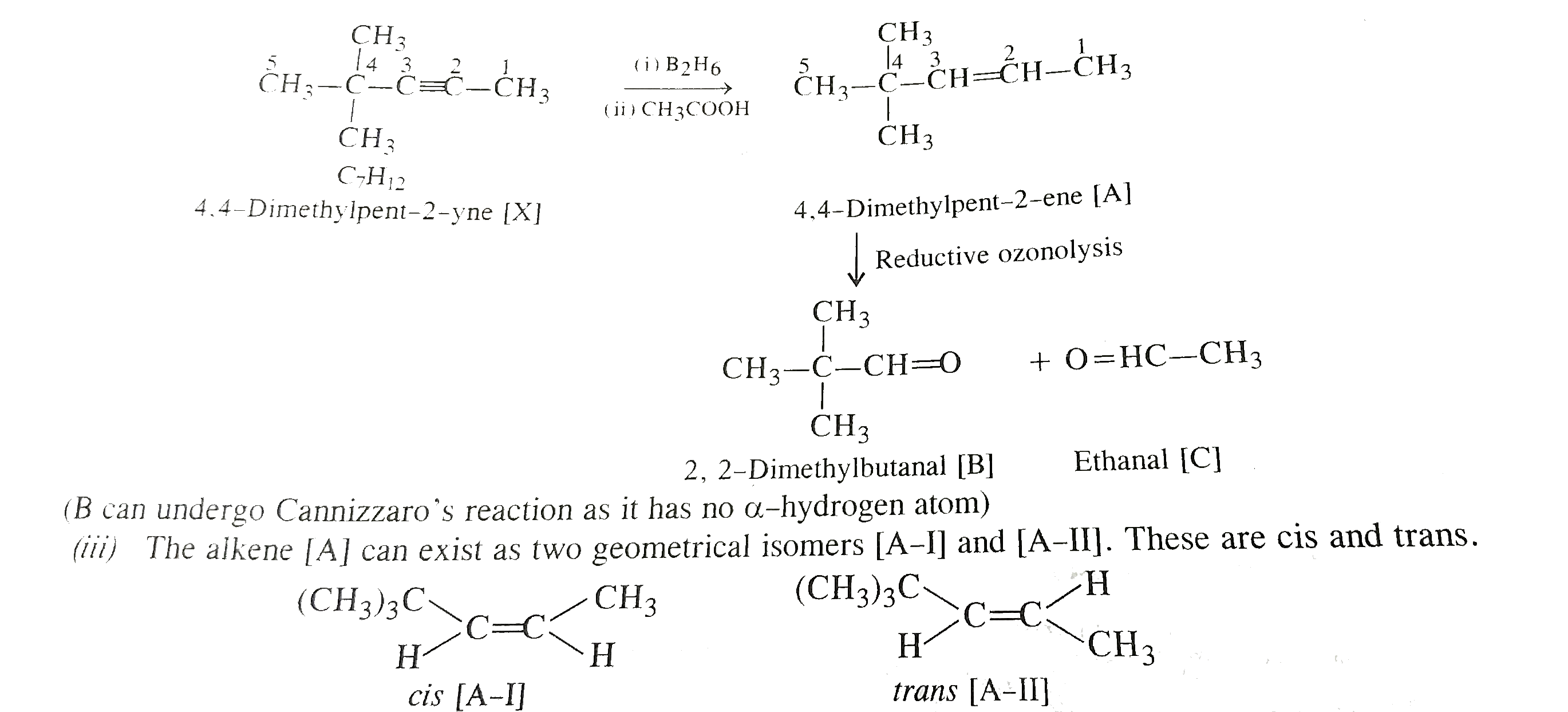Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Hydrocarbon (X), C(7)H(12), on reaction with boron hydride followed by...
Text Solution
|
- An organic compound (A) gives opsitive Liebermann reaction and on trea...
Text Solution
|
- Two isomeric compounds, (A) and (B), have the same formula, C(11)H(13)...
Text Solution
|
- Hydrocarbon (X), C(7)H(12), on reaction with boron hydride followed by...
Text Solution
|
- An unsaturated fatty acid on ozonolysis yields one aldehyde H(3)C(CH(2...
Text Solution
|
- An alkene (A) C(16)H(16) on ozonolysis gives only one product (B) (C(8...
Text Solution
|
- An alkane (A) C(16)H(16) on ozonolysisi gives only one products (B) C(...
Text Solution
|
- An organic compound (A) C(6)H(10) on reduction, first give (B) C(6)H(1...
Text Solution
|
- (A) overset(O(3))underset(Zn//AcOH)to(B) +(C ) C(7)H(14) Compound (A) ...
Text Solution
|