Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
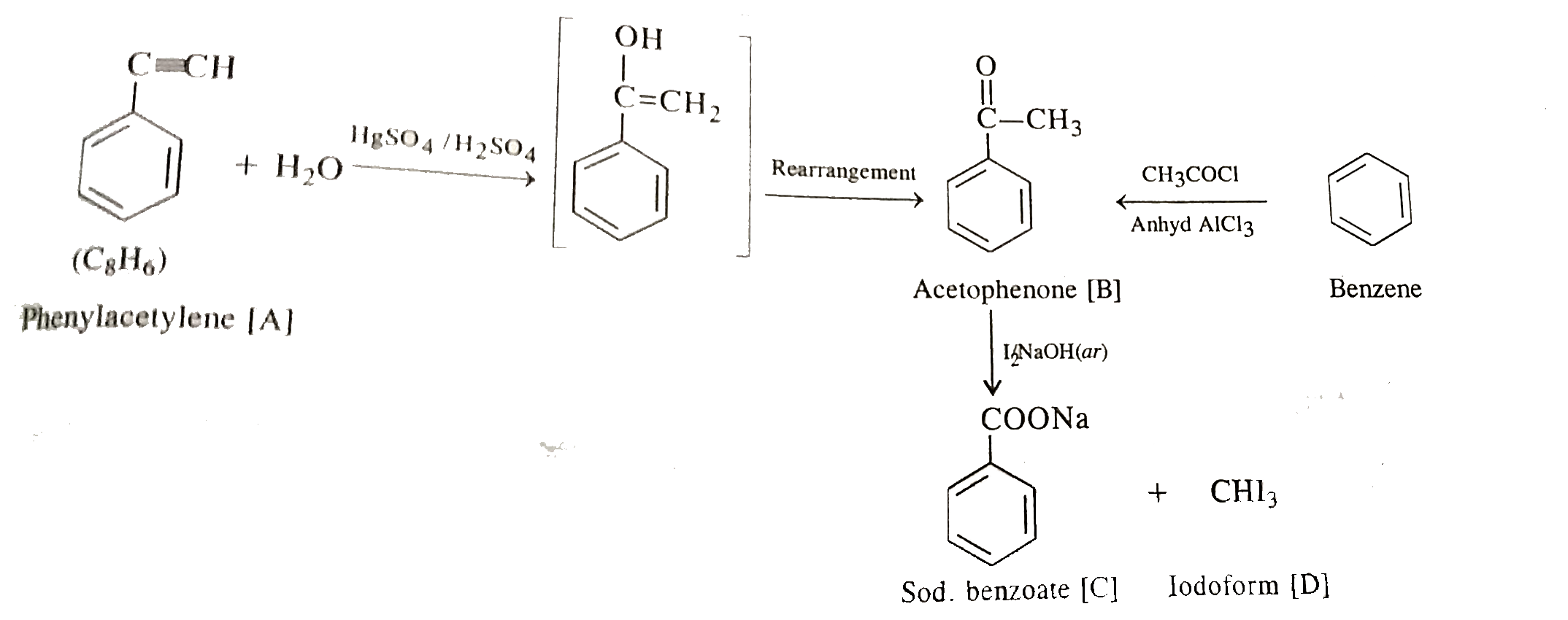
- An organic compound A, C(8)H(6) on reacting with dil. H(2)SO(4) and Hg...
Text Solution
|
- A compound X when passed through dil. H(2)SO(4) containing HgSO(4) giv...
Text Solution
|
- An organic compound A (C(2)H(6)O) reacts with sodium to form a compoun...
Text Solution
|
- An organic compound [A] C(8)H(6) on reacting with dilute sulphuric aci...
Text Solution
|
- An organic compound [A] is C(5)H(8)O(3). On heating with soda lime, it...
Text Solution
|
- An organic compound [A] is C(5)H(8)O(3). On heating with soda lime, it...
Text Solution
|
- An organic compound (A), C(8)H(4)O(3) in dry benzene in the presence o...
Text Solution
|
- Benzene reacts with CH(3)I in the presence of AlCl(3) to give compound...
Text Solution
|
- An organic compound A, C(8)H(6) on reacting with dil. H(2)SO(4) and Hg...
Text Solution
|