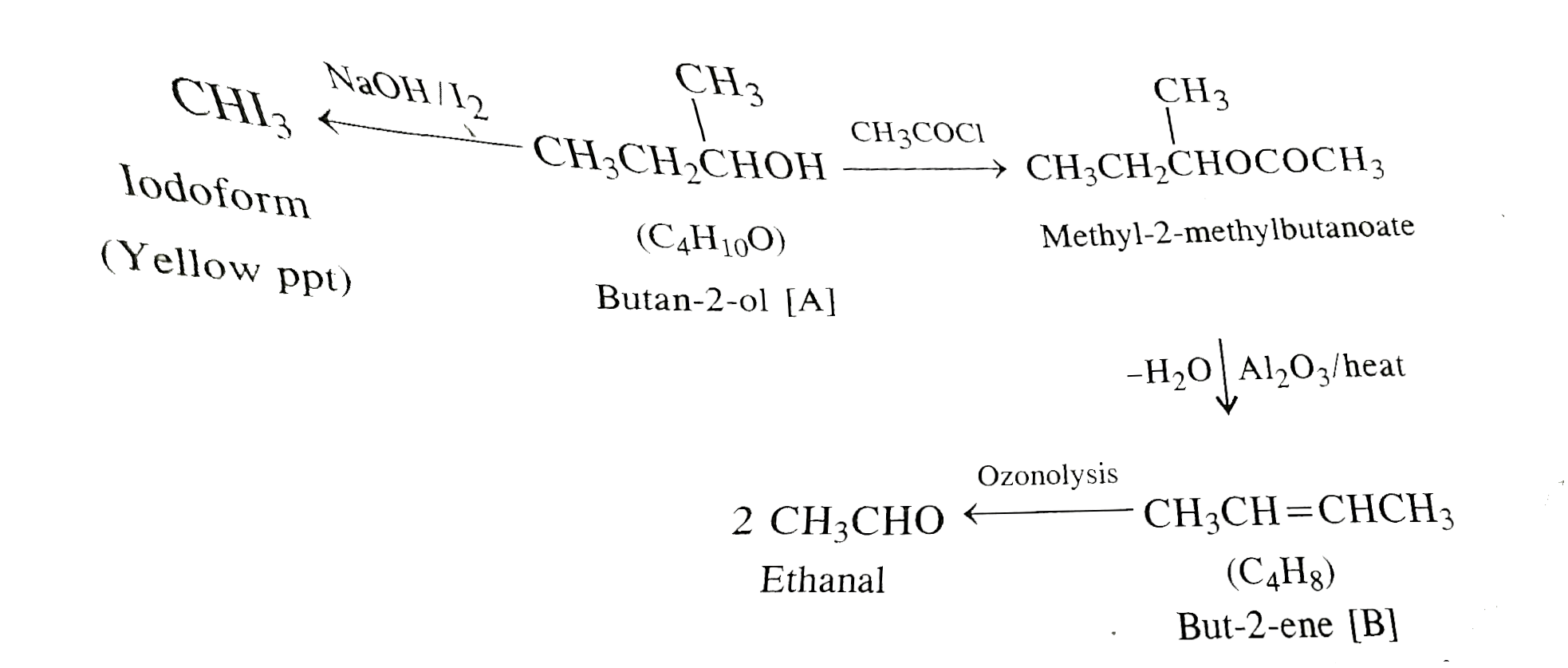Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Anorganic compound A (molecular formula C4H10O) reacts vigorously with...
Text Solution
|
- An organic compound 'A' (molecular formula C(4)H(10)O) reacts vigorusl...
Text Solution
|
- An alkene 'A' (molecular formula C(5)H(10)) on ozonolysis gives a mixt...
Text Solution
|
- An organic compound of molecular formula C4H10O does not react with so...
Text Solution
|
- An alkene (A) having formula C4H8, on ozonolysis, gives propanal & met...
Text Solution
|
- Compound (X) of molecular formula C4H8 takes up one equivalent of hydr...
Text Solution
|
- An organic compound (A) of molecular formula C4H8 is symmetric alkene....
Text Solution
|
- A compound (A) with molecular formula C4H10O on oxidation forms compou...
Text Solution
|
- An organic compound of molecular formula C4H10O does not react with so...
Text Solution
|