Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
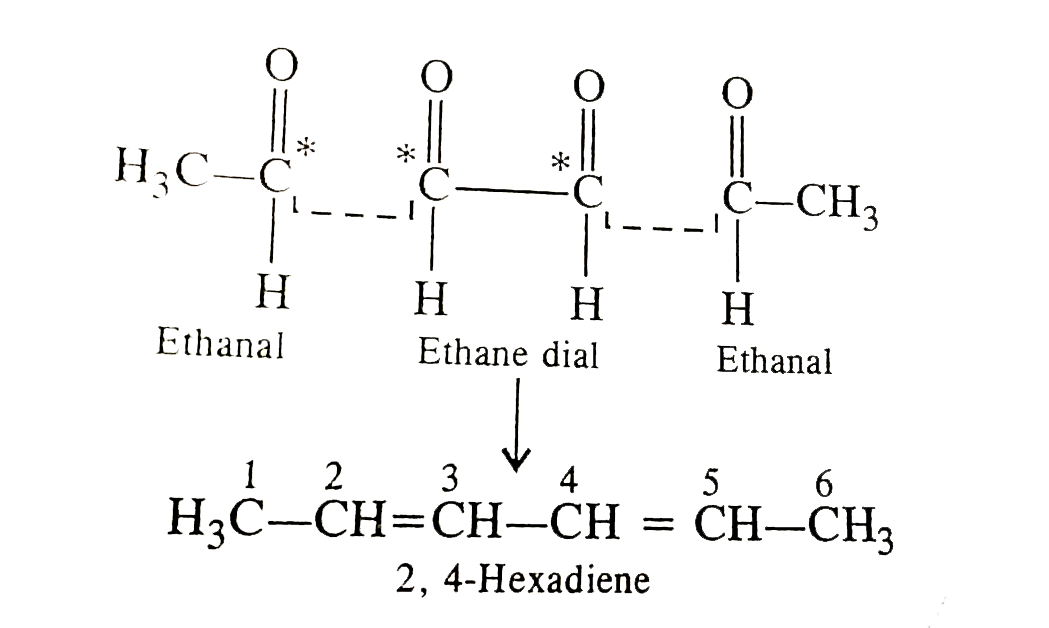
- Upon ozonolysis, molecule of a hydrcarbon produces of ethanal and one ...
Text Solution
|
- A diene on reductive ozonolysis produces two moles of ethanal and one ...
Text Solution
|
- An unsaturated hydrocarbon 'A' adds two molecules of H(2) and on reduc...
Text Solution
|
- An unsaturated hydrocarbon 'A' adds two molecules of H2 and on reducti...
Text Solution
|
- On ozonolysis , one mole of a hydrocarbon produces two molecules of et...
Text Solution
|
- An unsaturated hydrocarbon on ozonolysis gives one mole each of methan...
Text Solution
|
- एथेन (C2H6) के तीन मोलों में एथेन के अणुओं की संख्या का परिकलन कीजिए ...
Text Solution
|
- A hydrocarbon on ozonolysis produces ethanal and methanal. Identify th...
Text Solution
|
- One molecule of a hydrocarbon produces one molecule each of acetone, m...
Text Solution
|