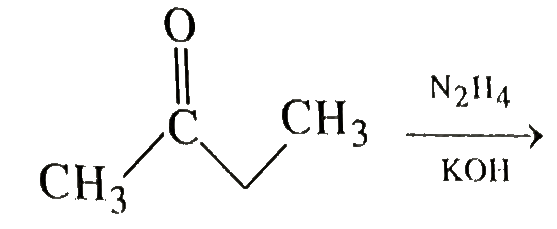
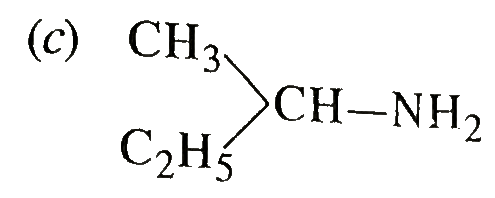
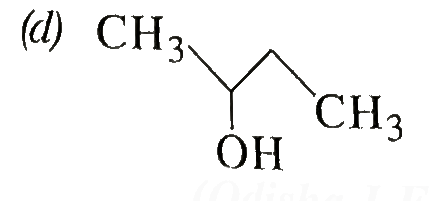
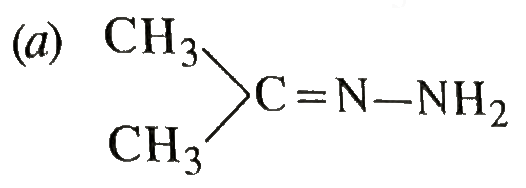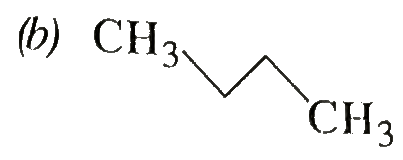A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The product of the solution :
Text Solution
|
- When an aqueous solution of H(2)SO(4) is electrolysed, the ion dischar...
Text Solution
|
- When the ionic product of a solution exceeds the solubility product, t...
Text Solution
|
- The product of the solution :
Text Solution
|
- When the ionic product of a solution exceeds the solubility product, t...
Text Solution
|
- The products obtained by passing chlorine through hypo solution are
Text Solution
|
- On warming an aqueous solution of benzenediazonium chloride, the produ...
Text Solution
|
- The emf of a cell with 1 M solutions of reactants and products in solu...
Text Solution
|
- On warming an aqueous solution of benzenediazonium chloride, the produ...
Text Solution
|