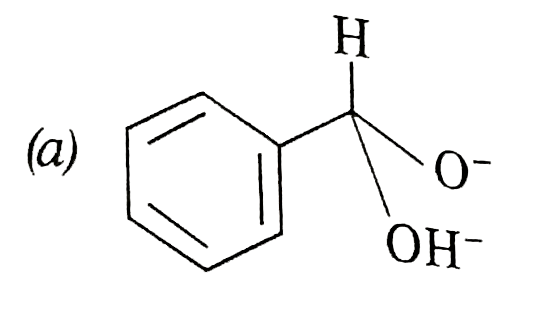
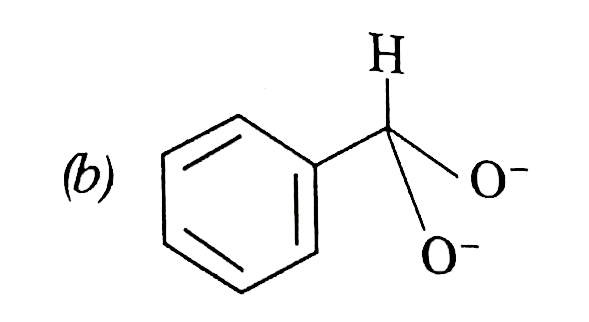
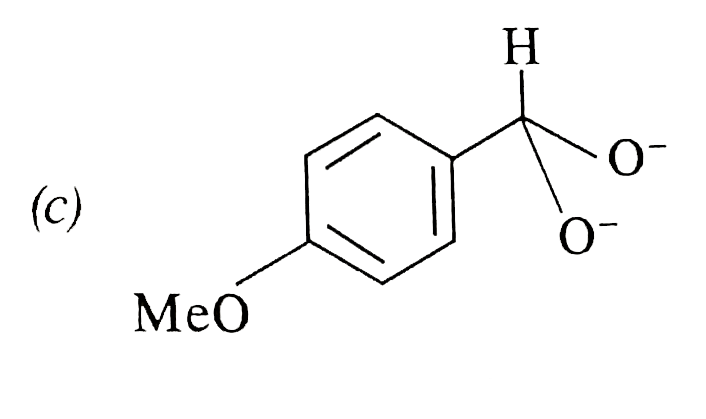
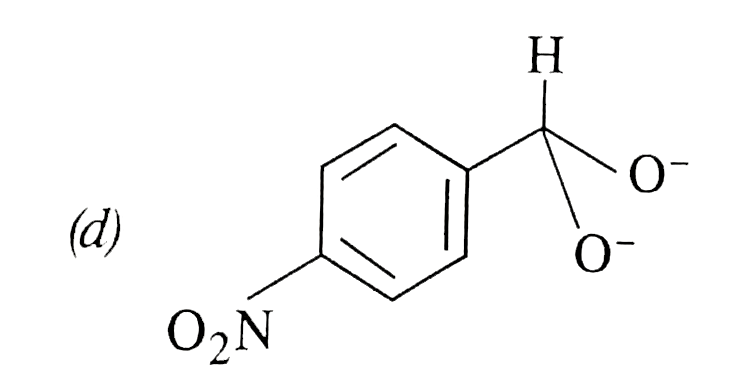
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In Cannizzaro's reaction, which intermediate ion is best hydride (H^(-...
Text Solution
|
- Among the following, the least and the best H^(Θ) ion donor, respectiv...
Text Solution
|
- Among the following, the best and the least H^(Θ) ion donor, respectiv...
Text Solution
|
- Among the following, the best and the least H^(Θ) ion donor, respectiv...
Text Solution
|
- In a Cannizaro reaction the intermediate that will be the best hydride...
Text Solution
|
- In Cannizzaro's reaction, which intermediate ion is best hydride (H^(-...
Text Solution
|
- In the cannizzaro's reaction the intermediate that will be the best hy...
Text Solution
|
- कैनिजारो अभिक्रिया में कौन-सा मध्यवर्ती सबसे अच्छा हाइड्राइड दानी है?
Text Solution
|
- In a Cannizzaro reaction, the intermediate that will be best hydride d...
Text Solution
|