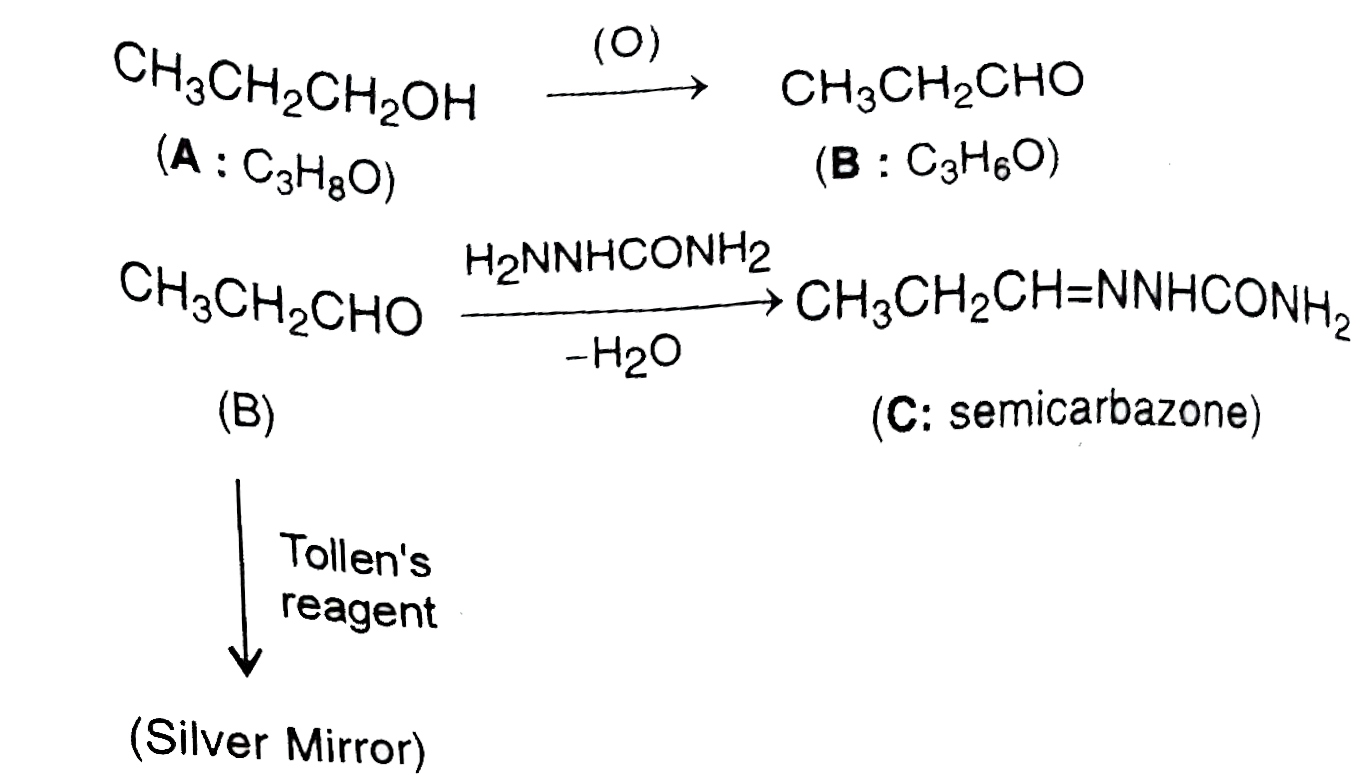A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compound (X) with molecular formula C(3)H(8)O is treated with acidifie...
Text Solution
|
- Compound (A) [molecular formula (C(3)H(8)O) ] is treated with acidifie...
Text Solution
|
- Compound A (molecular formula C(3)H(8)O)is treated with acidified pota...
Text Solution
|
- Compound 'A' (molecular formula C(3)H(8)O ) is treated with acidified ...
Text Solution
|
- Compound A (molecular formula C(3)H(8)O ) is treated with acidified po...
Text Solution
|
- Compound 'A' (molecular formula C(3)H(8)O) is treated with acidified p...
Text Solution
|
- Compound A (molecular formula C(3)H(8)O ) is trated with acidified pot...
Text Solution
|
- Compound A of formula C(3)H(8)O is treated with acidic KMnO(4) to form...
Text Solution
|
- Compound (X) with molecular formula C(3)H(8)O is treated with acidifie...
Text Solution
|