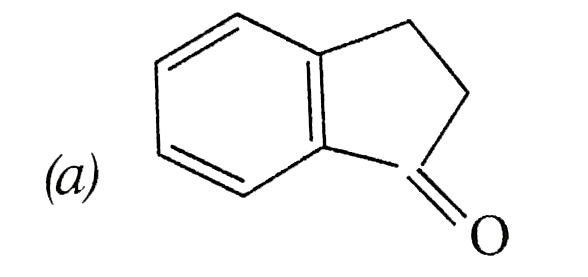
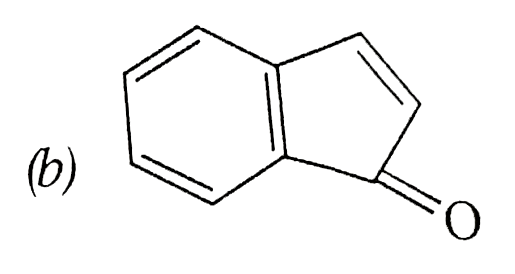
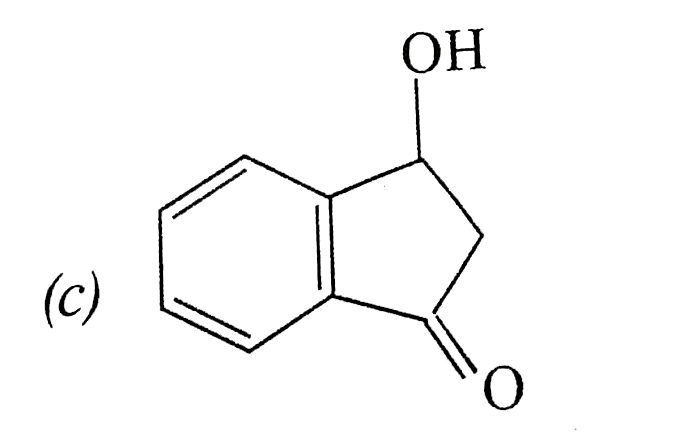
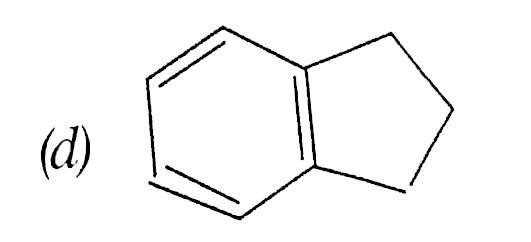
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Treatment of benzene with "CO"//HCl in the presence of anhydrous AlCl(...
Text Solution
|
- The reaction of compounf P with CH(3)MgBr (excess) in (C(2)H(5))(2)O f...
Text Solution
|
- Treatment of benzene with CO//HCl in the presence of anhydrous AlCl(3)...
Text Solution
|
- An organic compound (A), C(8)H(4)O(3) in dry benzene in the presence o...
Text Solution
|
- How many toluidines on reaction with NaNO(2)//HCl followed by H(3)PO(2...
Text Solution
|
- An organic compound A , C(4)H(4)O(3) in dry benzene in the presence of...
Text Solution
|
- How many toluidines on reaction with NaNO(2)//HCl followed by H(3)PO(2...
Text Solution
|
- Treatment of benzene with CO/HCl in the presence of anhydrous "AlCl"(3...
Text Solution
|
- Treatment of benzene with CO/HCl in the presence of anhydrous "AlCl"(3...
Text Solution
|