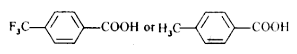Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which acid of each pair shown here would you expect to be stronger? ...
Text Solution
|
- For the following acids underset((I))((CH(3))(3)C CH(2)CO(2)H) " " und...
Text Solution
|
- End product of this conversion CH(3)-overset(O)overset(||)(C)-CH(2)-CH...
Text Solution
|
- {:("COlumn(I)","Column(II)"),("Acid",pK(a)),((a)CH(3)-CO(2)H,(p)5.69),...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger? ...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger ? ...
Text Solution
|
- Which is the most suitable reagent for the following transformation ? ...
Text Solution
|
- Which acid is stronger in each of the pairs of lower-acting acids? (i)...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger? ...
Text Solution
|