Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
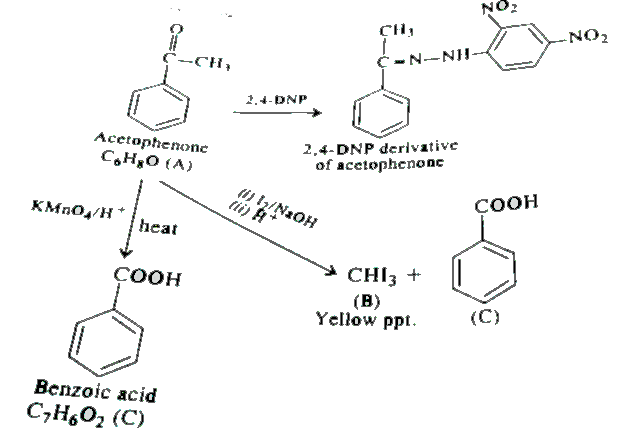
- An aromatic compound 'A' (molecular formula C(8)H(8)O) gives positive ...
Text Solution
|
- An aromatic compound A of the molecular formula C(8)H(10)O on reaction...
Text Solution
|
- An aromatic compound 'A' (Molecular formula C(8)H(8)O) ) gives positiv...
Text Solution
|
- An organic compound (A) with molecular formula C(8)H(8)O forms an oran...
Text Solution
|
- एक कार्बनिक यौगिक A (अणुसूत्र C(8)H(8)O), धनात्मक 2,4 - DNP परीक्षण...
Text Solution
|
- An aromatic compound (X) (C(8)H(8)O) gives positive 2, 4-DNP test. It ...
Text Solution
|
- An organic compound (A) with molecular formula C8H8 O forms an o...
Text Solution
|
- An aromatic compound 'A' (molecular formula C(8)H(8)O ) gives positive...
Text Solution
|
- एक कार्बनिक यौगिक 'A' अणुसूत्र C(8)H(8) O , धनत्मक 2 ,4 - DNP परीक्...
Text Solution
|