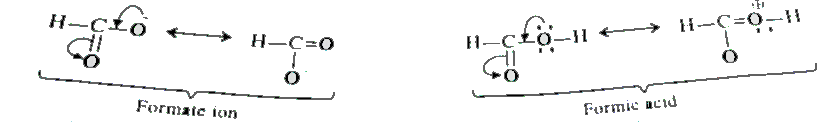Text Solution
Verified by Experts
Recommended Questions
- Give reasons for the following : 'Carbon-oxygen bond lengths in form...
Text Solution
|
- Give reasons for the following : 'Carbon-oxygen bond lengths in form...
Text Solution
|
- Cyclic hydrocarbon molecules 'A' has all the carbon and hydrogen in a ...
Text Solution
|
- Assertion: In sodium formate, both the C - O bonds have same value 1.2...
Text Solution
|
- If the bond length of CO bond in carbon monoxide is 1.128 Å , then wha...
Text Solution
|
- Carbon-oxygen bond lengths in formic acid are different but are the sa...
Text Solution
|
- Cyclic hydrocarbon molecules 'A' has all the carbon and hydrogen in a ...
Text Solution
|
- फॉर्मिक अम्ल में कार्बन-ऑक्सीजन बन्धों की लम्बाई 1.23 Å तथा 1.36 Å है...
Text Solution
|
- If the bond length of CO bond in carbon monoxide is 1.128 Å, then what...
Text Solution
|