Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
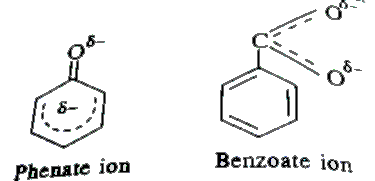
- Although phenoxide ion has more number of resonating structures than c...
Text Solution
|
- Although phenoxide ion has more number of resonating structures than c...
Text Solution
|
- Although phenoxide ion has more number of resonating structures than b...
Text Solution
|
- यद्पि फिनॉक्साइड आयन की अनुनादी संरचानए कार्बोक्सिलेट आयन की तुलना में...
Text Solution
|
- यद्यपि कार्बोक्सिलेट आयन की तुलना में फिनॉक्साइड आयन अधिक संख्या में अ...
Text Solution
|
- Although phenoxide ion has more number of resonating structures than c...
Text Solution
|
- यघपि फिनॉक्साइड आयन की अनुनादी संरचनाएँ कार्बोक्सिलेट आयन की तुलन...
Text Solution
|
- यघपि फिनॉक्साइड आयन की अनुनादी संरचनाएँ कार्बोक्सिलेट आयन की तुल...
Text Solution
|
- Although phenoxide ion has more number of resonating structures than c...
Text Solution
|