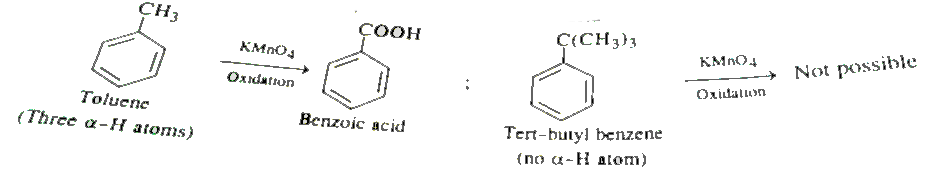
Text Solution
Verified by Experts
Recommended Questions
- Give reasons for the following : i. tertiary-Butyyl does not give be...
Text Solution
|
- Give reasons for the following: (i) tert-buty lbenzene does not give b...
Text Solution
|
- Give reason for the following in one or two sentences: 'Although ben...
Text Solution
|
- Benzene contains double bonds but does not give addition reactions bec...
Text Solution
|
- Tertiary butyl benzene does not give benzoic acid when oxidised with K...
Text Solution
|
- Assertion : Oxidation of toluene as well as ethyl benzene with KMnO(4)...
Text Solution
|
- Which of the followingon treatment withhot alkaline KMnO(4) gives benz...
Text Solution
|
- Normally, benzene gives electrophilic substitution reaction rather tha...
Text Solution
|
- Give reason for the following: (a) p-nitrophenol is more acidic than o...
Text Solution
|