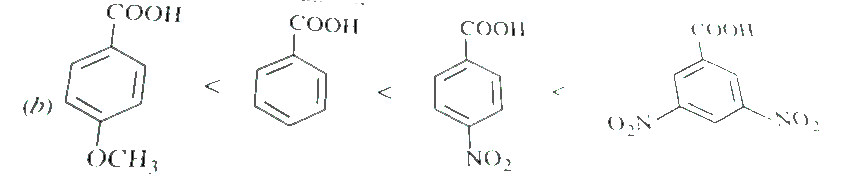
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Arrange the following compounds in order of increasing reactivity towa...
Text Solution
|
- Arrange the following compounds in order of increasing reactivity towa...
Text Solution
|
- Acetone forms bisulphite compound when heated with sodium bisulphate w...
Text Solution
|
- Acetone to tertiary butyl alcohol
Text Solution
|
- Arrange the Acetaldehyde, Acetone, Methyl t. butyl ketone reactivity t...
Text Solution
|
- Write the structures of the Di-Sec. butyl ketone compound.
Text Solution
|
- निम्नलिखित यौगिकों को उनमें संबंधित गुणधर्मो के बढ़ते क्रम में ...
Text Solution
|
- निम्न यौगिकों को उनसे संबंधित (कोष्ठकों में दिए गए) गुणधर्मो के बढ़ते क...
Text Solution
|
- Arrange the following compounds in increasing order of their property ...
Text Solution
|