Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
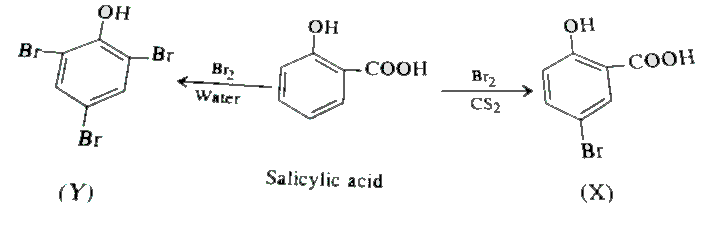
- Salicylic acid is treated with bromine under two different conditions ...
Text Solution
|
- The aciton of bromine water (excess) on salicylic acid results in the ...
Text Solution
|
- The action of bromine water (excess) on salicylic acid results in the ...
Text Solution
|
- Salicylic acid is treated with bromine under two different conditions ...
Text Solution
|
- Which reagent can produce salicylic acid from sodium phenoxide under s...
Text Solution
|
- When 2-hydroxybenzoic aicd (salicylic acid) is treated with bromine wa...
Text Solution
|
- How many of the following may produce salicylic acid on hydrolysis und...
Text Solution
|
- Salicylic acid on treatment with bromine water will give
Text Solution
|
- When salicylic acid is treated with acetic anhydride, is obtained .
Text Solution
|