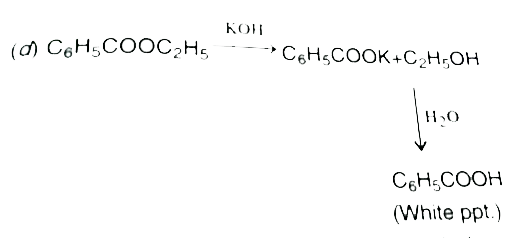A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- an ester is boiled with KOH. The product is cooled and acidified with ...
Text Solution
|
- An ester is boiled with KOH and the product is cooled and acidified wi...
Text Solution
|
- An ester is boiling with KOH. The product is cooled and acidified with...
Text Solution
|
- An organic compound is boiled with alcoholic potash. The product is co...
Text Solution
|
- an ester is boiled with KOH. The product is cooled and acidified with ...
Text Solution
|
- Acids have much higher boiling points than isomeric esters because :-
Text Solution
|
- Acids and esters are
Text Solution
|
- "Aclohols + Acids " overset(conc. H2SO4) to "Esters". This reaction is...
Text Solution
|
- किसी एस्टर को KOH के साथ उबला जाता है। उत्पाद को ठण्डा कर सांद्र HCl स...
Text Solution
|