A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Carboxylic acids have higher boiling points than aldehydes, ketones an...
Text Solution
|
- Carboxylic acid have higher boiling points than aldehydes, ketones an...
Text Solution
|
- कार्बोक्सिलिक अम्लों के क्वथनांक तुलनीय अणुभार युक्त ऐल्डिहाइड्स, कीटो...
Text Solution
|
- Carboxylic acids have higher boiling points than aldehydes, ketones an...
Text Solution
|
- लगभग समान अणुभार वाले ऐल्डिहाइड , कीटोन व ऐल्कोहॉल की तुलना में कार्बो...
Text Solution
|
- Carboxylic acids have higher boiling points than aldehydes, ketones an...
Text Solution
|
- कार्बोक्सिलिक अम्लें के क्वथनांक समतुल्य आण्विक द्रव्यमान वाले ऐल्ड...
Text Solution
|
- Carboxylic acids have higher boiling points than aldehydes, ketones an...
Text Solution
|
- Carboxylic acids have higher boiling points than aldehydes, ketones an...
Text Solution
|
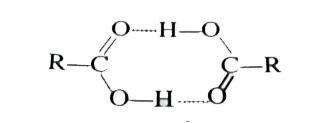 As a result, the boiling point of carboxylic acids is higher than those of the compounds listed.
As a result, the boiling point of carboxylic acids is higher than those of the compounds listed.