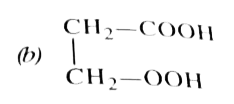
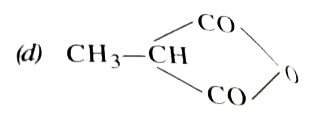
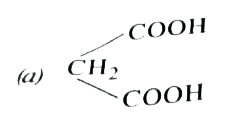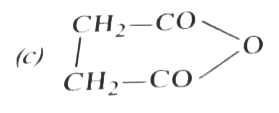A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- CH(3)-CO OH overset(Br(2)//P)to(A) overset(NaCN)to(B) overset(HOH//H^(...
Text Solution
|
- For the given reaction how many products are optically active ( all is...
Text Solution
|
- C(6)H(5)NH(2)overset((CH(3)CO)(2)O)rarrXunderset(C CI(4))overset(Br(2)...
Text Solution
|
- Ph-overset(O)overset(||)(C)-NH(2)+Ph-CH(2)-overset(O)overset(||)(C)- o...
Text Solution
|
- Total number of steroisomer of compound CH(3)-overset(OH)overset(|)(CH...
Text Solution
|
- The IUPAC name of CH(3)-overset(OH)overset(|)underset(H)underset(|)(C)...
Text Solution
|
- Me-CH(2)-CH(2)OH overset(KMnO(4)//H^(+))rarr (A)overset(Red P+Br(2))ra...
Text Solution
|
- Given the following sequence of reaction CH(3)CH(2)I overset(NaCN)(r...
Text Solution
|
- In the following sequence of reaction, the major product ( C) CH(3)...
Text Solution
|