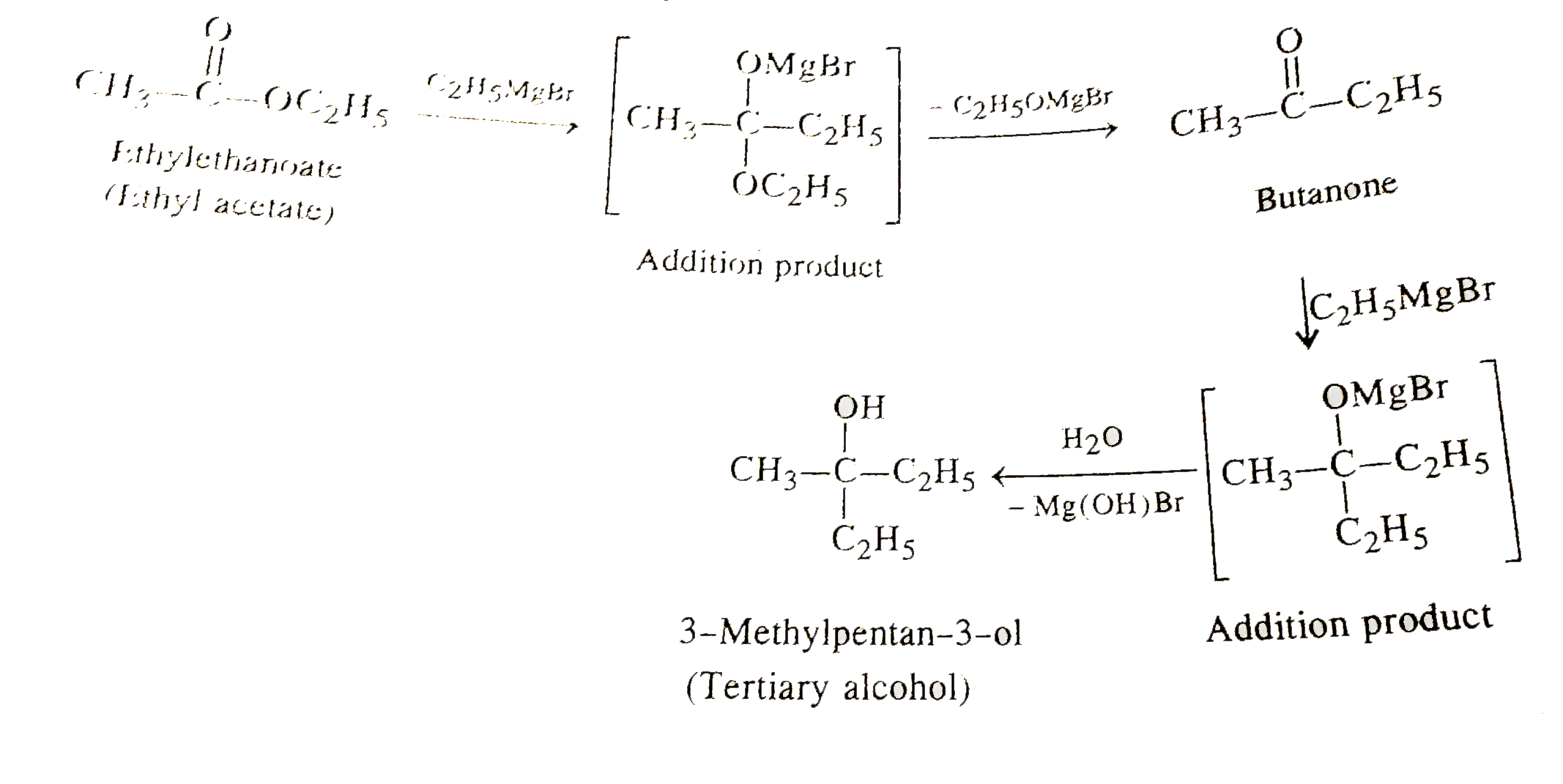Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write the structural formula of the main organic product formed when e...
Text Solution
|
- When ethyl ethanoate is treated with excess of MeMgBr, followed by hyd...
Text Solution
|
- Write the structural formula of the main organic product formed when e...
Text Solution
|
- Write the chemical eqution to show what happens when 'Ethyl acetate is...
Text Solution
|
- When ethyl bromide is treated with moist Ag(2)O main product is//are.
Text Solution
|
- With the help of chemical equations, write the structural formula of t...
Text Solution
|
- Indicate the expected structure of the organic product when ethyl magn...
Text Solution
|
- ऐथिल ब्रोमाइड की अभिक्रिया (a) AgNO(2) (ऐल्कोo) तथा (b) NaNO(2) के साथ...
Text Solution
|
- Indicate the expected structure of the organic product when ethyl magn...
Text Solution
|