Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
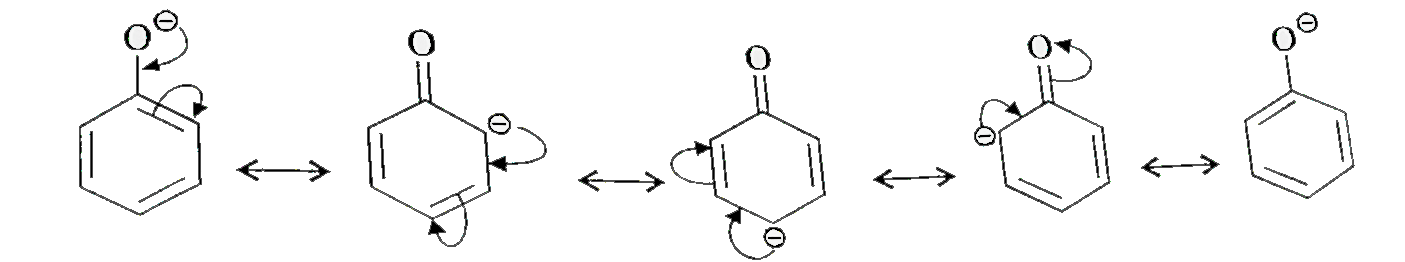
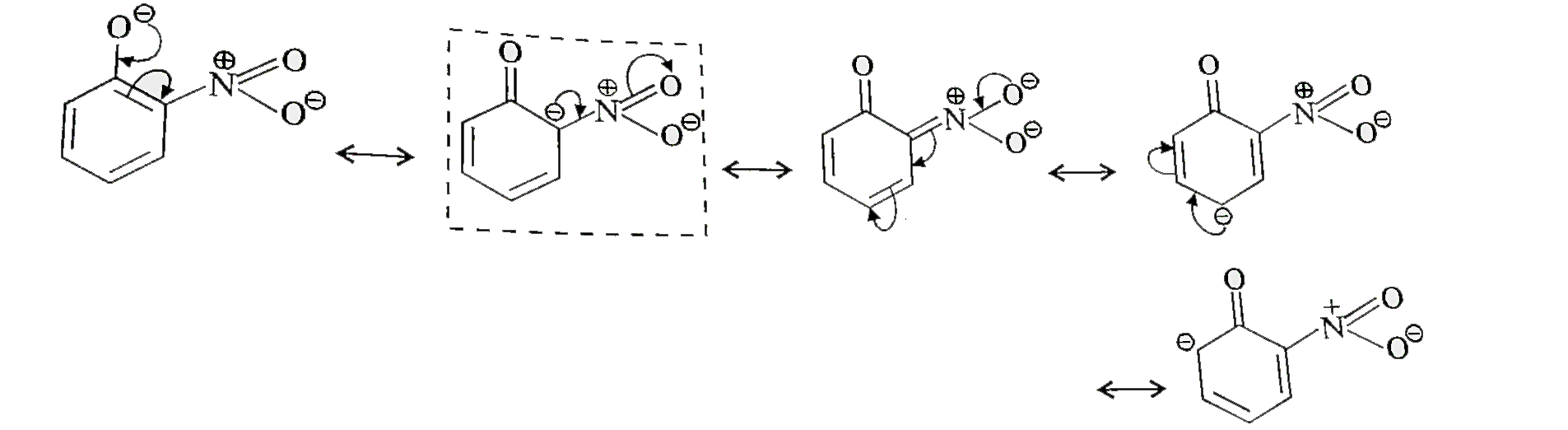
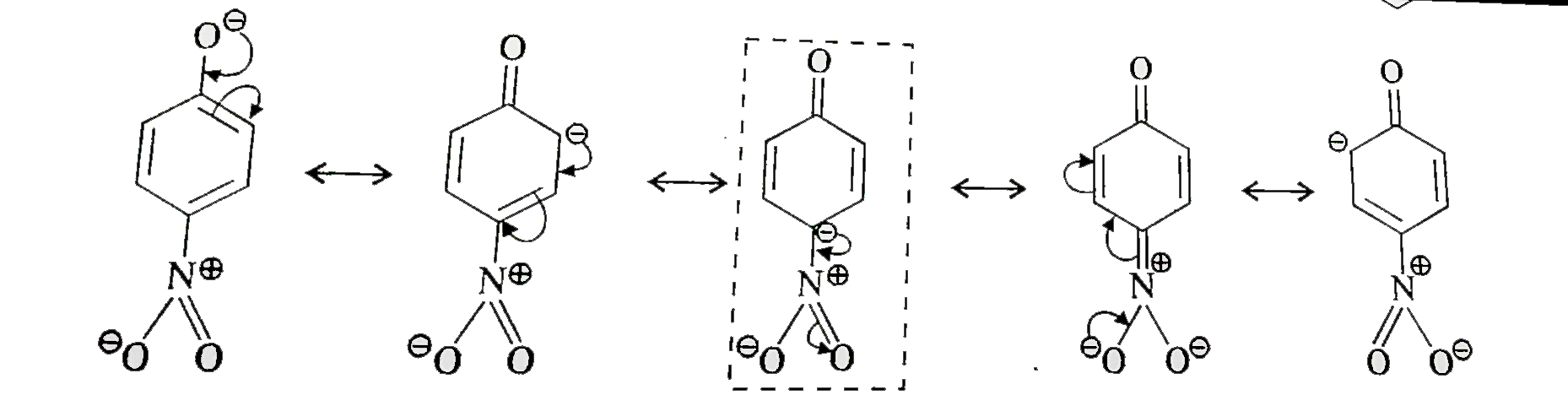
- Ortho and para nitrophenols are more acidic than phenol. Draw the reso...
Text Solution
|
- Ortho and para nitrophenols are more acidic than phenol. Draw the reso...
Text Solution
|
- Ortho and para nitrophenols are more acidic than phenol. Draw the reso...
Text Solution
|
- यद्पि फिनॉक्साइड आयन की अनुनादी संरचानए कार्बोक्सिलेट आयन की तुलना में...
Text Solution
|
- फिनॉल की तुलना में आर्थों तथा पैरा नाइट्रोफिनॉल ज्यादा अम्लीय है | संग...
Text Solution
|
- समझाइये क्यों आर्थों-नाइट्रोफिनॉल आर्थों-मिथाक्सी-फिनॉल से ज्यादा अम्ल...
Text Solution
|
- ऑर्थो तथा पेरनाइट्रोफीनॉल, फीनॉल से अधिक अम्लीय होती है ।उनके संगत फीन...
Text Solution
|
- Ortho and para nitrophenols are more acidic than phenol. Draw the reso...
Text Solution
|
- How many of these compounds are more acidic than phenol here. Formic a...
Text Solution
|